Research News
-
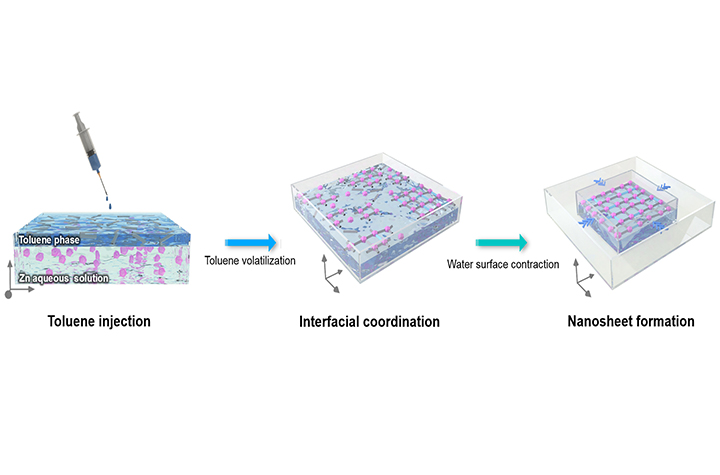 08 01, 2025Researchers Develop Triggered Interfacial Synthesis Strategy for Rapid Customization of Ultrathin 2D Metal-Organic Framework MembranesA research team led by Prof. YANG Weishen and Prof. PENG Yuan from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a triggered interfacial synthesis strategy that accelerates the fabrication of high-performance MOF membranes.Energy-efficient membrane technologies are essential for reducing energy consumption and carbon emissions in industrial separations. Two-dimensional metal-organic framework (2D MOF) molecular sieve membranes are promising due to their tunable structures, ultrathin thickness, and customizable pores - ideal for applications such as gas separation and deionization. However, their practical development has been severely hindered by time, consuming synthesis processes and inefficient assembly methods.In a study published in National Science Review, a research team led by Prof. YANG Weishen and Prof. PENG Yuan from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a triggered interfacial synthesis strategy that accelerates the fabrication of high-performance MOF membranes.Water surface contraction boosted the air-water interfacial coordination strategy for the most rapid fabrication record of 2D MOF membranes (Image by Peng Yuan)The strategy combines confined air–water interfacial nanosheet synthesis with a radial thrust-triggered in situ assembly, reducing fabrication time from hours or days to just minutes. The process requires only microliter-scale consumption of organic ligands, offering a cost-effective and scalable pathway.Using this strategy, researchers fabricated a Zn-MOF nanosheet membrane that achieved a H2 and CO2 separation factor of 210 and hydrogen permeance of 6.2 × 10−7 mol/m2•s•Pa, outperforming both conventional MOF membranes and commercial alteratives. These results represent state-of-the-art performance and demonstrate the feasibility of on-demand design of ultrathin 2D MOF membranes for industrial applications.Beyond membrane performance, this strategy exhibits remarkable versatility. By flexibly combining different metal ions and organic ligands, the researchers synthesized 12 types of MOF nanosheets with distinct framework structures and pore/channel environments. These results provide a novel approach for the application-oriented and customizable fabrication across a wide range of eparation scenarios."This work not only provides a new strategy for efficiently customization of MOF nanosheets, but also expands the application potential of the ultrathin, flexible 2D materials with high-density, regular pore arrays in material science, device architecture design, separation engineering," said Prof. YANG.
08 01, 2025Researchers Develop Triggered Interfacial Synthesis Strategy for Rapid Customization of Ultrathin 2D Metal-Organic Framework MembranesA research team led by Prof. YANG Weishen and Prof. PENG Yuan from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a triggered interfacial synthesis strategy that accelerates the fabrication of high-performance MOF membranes.Energy-efficient membrane technologies are essential for reducing energy consumption and carbon emissions in industrial separations. Two-dimensional metal-organic framework (2D MOF) molecular sieve membranes are promising due to their tunable structures, ultrathin thickness, and customizable pores - ideal for applications such as gas separation and deionization. However, their practical development has been severely hindered by time, consuming synthesis processes and inefficient assembly methods.In a study published in National Science Review, a research team led by Prof. YANG Weishen and Prof. PENG Yuan from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a triggered interfacial synthesis strategy that accelerates the fabrication of high-performance MOF membranes.Water surface contraction boosted the air-water interfacial coordination strategy for the most rapid fabrication record of 2D MOF membranes (Image by Peng Yuan)The strategy combines confined air–water interfacial nanosheet synthesis with a radial thrust-triggered in situ assembly, reducing fabrication time from hours or days to just minutes. The process requires only microliter-scale consumption of organic ligands, offering a cost-effective and scalable pathway.Using this strategy, researchers fabricated a Zn-MOF nanosheet membrane that achieved a H2 and CO2 separation factor of 210 and hydrogen permeance of 6.2 × 10−7 mol/m2•s•Pa, outperforming both conventional MOF membranes and commercial alteratives. These results represent state-of-the-art performance and demonstrate the feasibility of on-demand design of ultrathin 2D MOF membranes for industrial applications.Beyond membrane performance, this strategy exhibits remarkable versatility. By flexibly combining different metal ions and organic ligands, the researchers synthesized 12 types of MOF nanosheets with distinct framework structures and pore/channel environments. These results provide a novel approach for the application-oriented and customizable fabrication across a wide range of eparation scenarios."This work not only provides a new strategy for efficiently customization of MOF nanosheets, but also expands the application potential of the ultrathin, flexible 2D materials with high-density, regular pore arrays in material science, device architecture design, separation engineering," said Prof. YANG. -
 07 29, 2025Dalian Advanced Light Source Achieves Key Progress in High-Repetition-Rate Electron Beam OperationA research team led by Academician YANG Xueming and Prof. ZHANG Weiqing from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), achieved stable operation of an electron beam at a repetition rate of 1 Megahertz (1 MHz) for the first time.On July 24, a research team led by Academician YANG Xueming and Prof. ZHANG Weiqing from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), achieved stable operation of an electron beam at a repetition rate of 1 Megahertz (1 MHz) for the first time. This result marks a meaningful step forward for the Dalian Advanced Light Source (DALS) project.To support the development of DALS—a next-generation extreme ultraviolet free-electron laser (EUV FEL)—the Dalian municipal government launched a prototype initiative focused on building a superconducting continuous-wave (CW) electron injector. Launched in 2020, the project involves DICP and the Institute of Advanced Light Source Facilities in Shenzhen. Its main objective is to overcome the core technical challenges associated with generating high-repetition-rate, high-quality electron beams, and a prerequisite for future high-average-power FEL facilities.Over the past five years, the team has advanced a series of key technologies, including a photocathode electron gun, ultrafast laser systems, solid-state radio-frequency (RF) power sources, superconducting accelerator modules, and cryogenic helium refrigeration systems. Earlier this year, the installation and individual system commissioning were completed. Following two weeks of integrated testing, the team began beam experiments at the megahertz scale and, at 11:15 p.m. on July 24, achieved stable beam operation.The current test platform now supports acceleration of an electron beam with an average current of 0.1 milliamperes. Key performance indicators include beam energy exceeding 100 mega-electron-volts, a single-pulse charge above 100 picocoulombs, and a repetition rate of 1 MHz. The system has demonstrated stable operation for more than one hour, with beam energy stability maintained below 0.01% root-mean-square.Achieving high-repetition-rate, high-quality electron beams is one of the most formidable technical challenges in building DALS. The success of this experiment demonstrates that the joint team has now gained critical expertise in the core enabling technologies, laying a strong foundation for future construction of DALS.
07 29, 2025Dalian Advanced Light Source Achieves Key Progress in High-Repetition-Rate Electron Beam OperationA research team led by Academician YANG Xueming and Prof. ZHANG Weiqing from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), achieved stable operation of an electron beam at a repetition rate of 1 Megahertz (1 MHz) for the first time.On July 24, a research team led by Academician YANG Xueming and Prof. ZHANG Weiqing from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), achieved stable operation of an electron beam at a repetition rate of 1 Megahertz (1 MHz) for the first time. This result marks a meaningful step forward for the Dalian Advanced Light Source (DALS) project.To support the development of DALS—a next-generation extreme ultraviolet free-electron laser (EUV FEL)—the Dalian municipal government launched a prototype initiative focused on building a superconducting continuous-wave (CW) electron injector. Launched in 2020, the project involves DICP and the Institute of Advanced Light Source Facilities in Shenzhen. Its main objective is to overcome the core technical challenges associated with generating high-repetition-rate, high-quality electron beams, and a prerequisite for future high-average-power FEL facilities.Over the past five years, the team has advanced a series of key technologies, including a photocathode electron gun, ultrafast laser systems, solid-state radio-frequency (RF) power sources, superconducting accelerator modules, and cryogenic helium refrigeration systems. Earlier this year, the installation and individual system commissioning were completed. Following two weeks of integrated testing, the team began beam experiments at the megahertz scale and, at 11:15 p.m. on July 24, achieved stable beam operation.The current test platform now supports acceleration of an electron beam with an average current of 0.1 milliamperes. Key performance indicators include beam energy exceeding 100 mega-electron-volts, a single-pulse charge above 100 picocoulombs, and a repetition rate of 1 MHz. The system has demonstrated stable operation for more than one hour, with beam energy stability maintained below 0.01% root-mean-square.Achieving high-repetition-rate, high-quality electron beams is one of the most formidable technical challenges in building DALS. The success of this experiment demonstrates that the joint team has now gained critical expertise in the core enabling technologies, laying a strong foundation for future construction of DALS. -
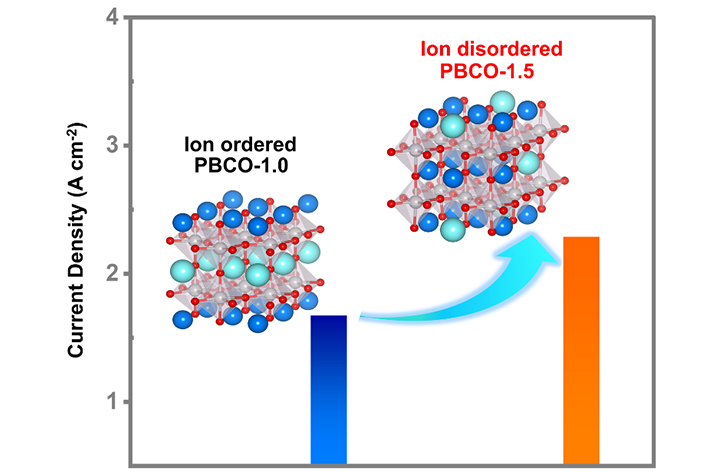 07 28, 2025Researchers Uncover Role of A-site Cation Ordering in Perovskite Anodes for High-temperature Oxygen EvolutionResearchers uncovered the mechanisms underlying the anodic high-temperature OER in SOECs.Solid oxide electrolysis cells (SOECs) are a leading technology for carbon dioxide reduction and energy conversion, offering high current densities, excellent Faradaic efficiency, and low overpotentials. Perovskite oxides are commonly used as SOEC anodes, yet the impact of A-site cation ordering on their oxygen evolution reaction (OER) kinetics remains unexplored.In a study published in Journal of the American Chemical Society, Associate Prof. SONG Yuefeng and colleagues from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. WANG Guoxiong from Fudan University and Prof. LIU Meilin from Georgia Institute of Technology, uncovered the mechanisms underlying the anodic high-temperature OER in SOECs.The relationship between the ion ordering and high-temperature OER activity (Image by YU Lina)Researchers focused on how A-site cation ordering affects the electrocatalytic performance of perovskite anodes, and particularly examined the order–disorder transition in PrxBa2-xCo2O5+δ.Researchers synthesized two perovskite anodes with different Pr contents, PrBaCo2O5+δ (PBCO-1.0) and Pr1.5Ba0.5Co2O5+δ (PBCO-1.5), and systematically investigated the effect of A-site cation ordering on the electronic structure and high-temperature OER kinetics.They found that as the Pr content increased from 1.0 to 1.5, the crystal structure transitioned from an ordered tetragonal phase (P4/mmm) to a disordered orthorhombic phase (Pnma). This structural transformation disrupted the local symmetry of the Co-O coordination. It enhanced the orbital hybridization between Co 3d and O 2p states, and improved oxygen ion mobility, ultimately accelerating surface oxygen exchange.At 800 °C and 1.6 V, the PBCO-1.5 anode delivered a high current density of 2.29 A cm-2, demonstrating good high-temperature OER activity and stability."Our study combines experimental data with theoretical insights to show how A-site cation ordering in perovskite oxides governs the reaction pathway and kinetics of high-temperature OER," said Assoc. Prof. SONG. "The findings provide valuable guidance for the rational design of high-performance SOEC anodes."
07 28, 2025Researchers Uncover Role of A-site Cation Ordering in Perovskite Anodes for High-temperature Oxygen EvolutionResearchers uncovered the mechanisms underlying the anodic high-temperature OER in SOECs.Solid oxide electrolysis cells (SOECs) are a leading technology for carbon dioxide reduction and energy conversion, offering high current densities, excellent Faradaic efficiency, and low overpotentials. Perovskite oxides are commonly used as SOEC anodes, yet the impact of A-site cation ordering on their oxygen evolution reaction (OER) kinetics remains unexplored.In a study published in Journal of the American Chemical Society, Associate Prof. SONG Yuefeng and colleagues from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. WANG Guoxiong from Fudan University and Prof. LIU Meilin from Georgia Institute of Technology, uncovered the mechanisms underlying the anodic high-temperature OER in SOECs.The relationship between the ion ordering and high-temperature OER activity (Image by YU Lina)Researchers focused on how A-site cation ordering affects the electrocatalytic performance of perovskite anodes, and particularly examined the order–disorder transition in PrxBa2-xCo2O5+δ.Researchers synthesized two perovskite anodes with different Pr contents, PrBaCo2O5+δ (PBCO-1.0) and Pr1.5Ba0.5Co2O5+δ (PBCO-1.5), and systematically investigated the effect of A-site cation ordering on the electronic structure and high-temperature OER kinetics.They found that as the Pr content increased from 1.0 to 1.5, the crystal structure transitioned from an ordered tetragonal phase (P4/mmm) to a disordered orthorhombic phase (Pnma). This structural transformation disrupted the local symmetry of the Co-O coordination. It enhanced the orbital hybridization between Co 3d and O 2p states, and improved oxygen ion mobility, ultimately accelerating surface oxygen exchange.At 800 °C and 1.6 V, the PBCO-1.5 anode delivered a high current density of 2.29 A cm-2, demonstrating good high-temperature OER activity and stability."Our study combines experimental data with theoretical insights to show how A-site cation ordering in perovskite oxides governs the reaction pathway and kinetics of high-temperature OER," said Assoc. Prof. SONG. "The findings provide valuable guidance for the rational design of high-performance SOEC anodes." -
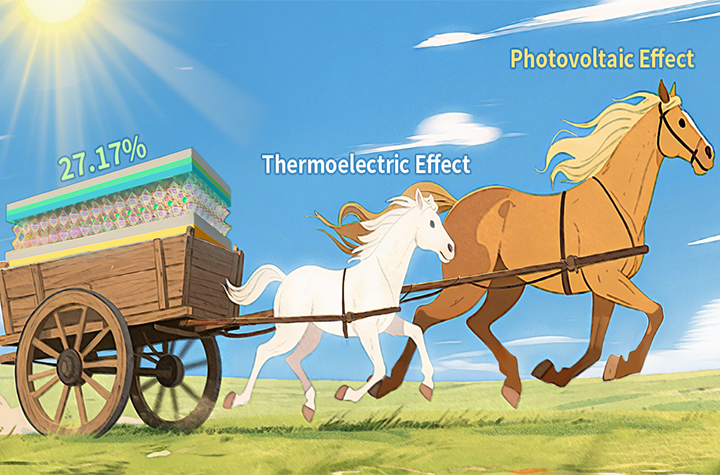 07 14, 2025Researchers Demonstrate Synergistic Cooperation between Photovoltaic and Thermoelectric Effects to Boost Solar Cell EfficiencyProf. LI Can’s research team has developed a novel perovskite solar cell that synergistically combines photovoltaic (PV) and thermoelectric (TE) effects to harvest both light and heat.Conventional photovoltaic (PV) cells are fundamentally limited by the Shockley–Queisser (SQ) efficiency limit, largely due to their inability to convert long-wavelength infrared photons into electricity. Unlocking this untapped infrared spectrum remains a key challenge for achieving maximal solar energy conversion efficiency.While thermoelectric (TE) effects offer a theoretical pathway to convert thermal energy into electrical power, the integration of PV and TE effects within a single PV cell has remained largely unexplored. Key questions persist:Does the TE effect manifest in the PV cells? What are the underlying factors linking PV and TE effects that possibly contribute to enhancing the power conversion efficiency (PCE) of the PV cells?In a study published in Energy & Environmental Science, a research team led by Prof. Can Li from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has addressed this challenge through synergizing PV and TE effects within a single perovskite solar cells.Leveraging the low thermal conductivity and intrinsic thermoelectric properties of perovskite materials, the researchers engineered a vertical temperature gradient (ΔT) within the perovskite solar cell. This design simultaneously exploits two mechanisms: short-wavelength photons are converted via the PV effect, while long-wavelength infrared energy—typically lost as heat—is harvested through the TE effect.By optimizing device architecture and charge transport dynamics, the researchers experimentally demonstrated a synergistic interaction between photogenerated carriers (PV effect) and thermally diffused carriers (TE effect). The integrated approach achieved a PCE of 27.17% at ΔT=10°C in the FAPbI₃-based solar cell— surpassing the baseline PCE of 25.65%. The enhanced PCE is attributed to broader spectral utilization and directional charge carrier transport induced by the engineered temperature gradient, which facilitates more efficient carrier collection.Our findings demonstrate the synergistic cooperation betweenPV and TE effects in enhancing the performance of PSCs. Looking forward, this strategy holds particular promise for environments with natural temperature gradients—such as marine settings, where cold seawater lies beneath warmer air, or near-space and polar regions, where intense solar irradiation coincides with low ambient temperatures."This work experimentally confirms the feasibility of integrating photovoltaic and thermoelectric effects for simultaneous light and heat harvesting, presenting a viable strategy for next-generation high-performance solar technologies." said Prof. LI.
07 14, 2025Researchers Demonstrate Synergistic Cooperation between Photovoltaic and Thermoelectric Effects to Boost Solar Cell EfficiencyProf. LI Can’s research team has developed a novel perovskite solar cell that synergistically combines photovoltaic (PV) and thermoelectric (TE) effects to harvest both light and heat.Conventional photovoltaic (PV) cells are fundamentally limited by the Shockley–Queisser (SQ) efficiency limit, largely due to their inability to convert long-wavelength infrared photons into electricity. Unlocking this untapped infrared spectrum remains a key challenge for achieving maximal solar energy conversion efficiency.While thermoelectric (TE) effects offer a theoretical pathway to convert thermal energy into electrical power, the integration of PV and TE effects within a single PV cell has remained largely unexplored. Key questions persist:Does the TE effect manifest in the PV cells? What are the underlying factors linking PV and TE effects that possibly contribute to enhancing the power conversion efficiency (PCE) of the PV cells?In a study published in Energy & Environmental Science, a research team led by Prof. Can Li from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has addressed this challenge through synergizing PV and TE effects within a single perovskite solar cells.Leveraging the low thermal conductivity and intrinsic thermoelectric properties of perovskite materials, the researchers engineered a vertical temperature gradient (ΔT) within the perovskite solar cell. This design simultaneously exploits two mechanisms: short-wavelength photons are converted via the PV effect, while long-wavelength infrared energy—typically lost as heat—is harvested through the TE effect.By optimizing device architecture and charge transport dynamics, the researchers experimentally demonstrated a synergistic interaction between photogenerated carriers (PV effect) and thermally diffused carriers (TE effect). The integrated approach achieved a PCE of 27.17% at ΔT=10°C in the FAPbI₃-based solar cell— surpassing the baseline PCE of 25.65%. The enhanced PCE is attributed to broader spectral utilization and directional charge carrier transport induced by the engineered temperature gradient, which facilitates more efficient carrier collection.Our findings demonstrate the synergistic cooperation betweenPV and TE effects in enhancing the performance of PSCs. Looking forward, this strategy holds particular promise for environments with natural temperature gradients—such as marine settings, where cold seawater lies beneath warmer air, or near-space and polar regions, where intense solar irradiation coincides with low ambient temperatures."This work experimentally confirms the feasibility of integrating photovoltaic and thermoelectric effects for simultaneous light and heat harvesting, presenting a viable strategy for next-generation high-performance solar technologies." said Prof. LI. -
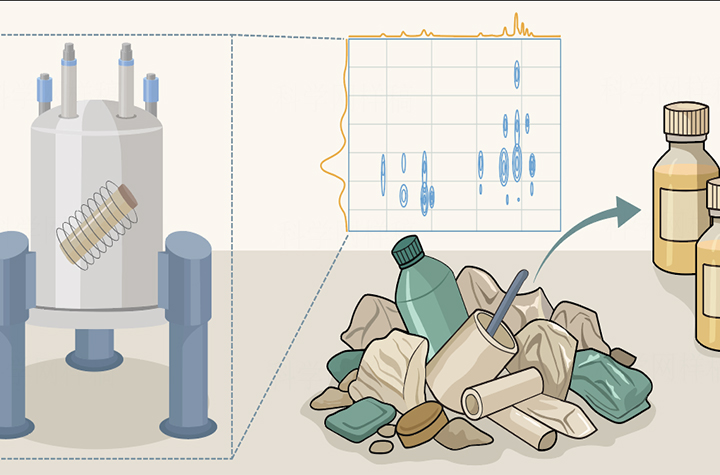 06 26, 2025In-line NMR Guides Orthogonal Transformation of Real-life PlasticsResearchers reported a advance in the indentification, separation, and catalytic transformation of real-life plastic waste mixtures.The global accumulation of plastic waste poses a serious threat to wildlife and ecosystems. Catalytic processes that convert plastic waste into valuable chemicals and fuels offer a promising solution. However, the recycling of real-life plastic waste mixtures remains a challenge due to their highly diverse composition and structural complexity. Accurate identification of the components within plastic waste mixtures is a prerequisite for their effective separation and recycling.Solid-state nuclear magnetic resonance (NMR) spectroscopy, which has the advantage of directly analyzing insoluble samples, provides detailed information into local atomic structure, molecular motion, interactions, and chemical environment—making it a powerful tool for studying complex polymer systems.In a study published in Nature, Prof. XU Shutao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with the team of Prof. WANG Meng and Prof. MA Ding from Peking University, reported a advance in the indentification, separation, and catalytic transformation of real-life plastic waste mixtures.The researchers utilized an innovative solid-state NMR method—1H-13C Frequency Switched Lee Goldburg Heteronuclear Correlation (FSLG-HETCOR) NMR. By optimizing key parameters such as spinning rate, contact time, and homonuclear decoupling field strength, and using 13C-labeled tyrosine hydrochloride as a reference, the researchers obtained high-resolution “fingerprint” spectra of individual plastic components from an eight-plastic mixture containing PS (polystyrene), PLA (polylactic acid), PU (polyurethane), PC (polycarbonate), PVC (polyvinyl chloride), PET (polyethylene terephthalate), PE (polyethylene), and PP (polypropylene).The method obtained spectra with high signal intensity and good resolution in the indirect dimension, allowing for precise identification of various functional groups in the plastic mixture and enabling real-time tracking of their chemical evolution.Furthermore, the researchers demonstrated the feasibility, effectiveness, and universality of this method by monitoring the full catalytic separation and transformation of real-life plastic waste mixtures. Their NMR-based analysis enabled the mapping of each step in the conversion process—from complex mixtures to multiple high-value chemicals products.By identifying characteristic functional group signals in plastic waste mixtures, the researchers laid a solid foundation for their effective separation and transformation. This work paves the way for integrating existing transformation processes into a unified framework, providing technical support for scalable industrial solutions to global plastic pollution."Solid-state NMR provides a powerful way to identify individual components in plastic waste mixtures. It acts as a 'guiding eye' for the separation and catalytic transformation processes, laying the technological foundation for real-life solutions to plastic pollution," said Prof. XU.
06 26, 2025In-line NMR Guides Orthogonal Transformation of Real-life PlasticsResearchers reported a advance in the indentification, separation, and catalytic transformation of real-life plastic waste mixtures.The global accumulation of plastic waste poses a serious threat to wildlife and ecosystems. Catalytic processes that convert plastic waste into valuable chemicals and fuels offer a promising solution. However, the recycling of real-life plastic waste mixtures remains a challenge due to their highly diverse composition and structural complexity. Accurate identification of the components within plastic waste mixtures is a prerequisite for their effective separation and recycling.Solid-state nuclear magnetic resonance (NMR) spectroscopy, which has the advantage of directly analyzing insoluble samples, provides detailed information into local atomic structure, molecular motion, interactions, and chemical environment—making it a powerful tool for studying complex polymer systems.In a study published in Nature, Prof. XU Shutao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with the team of Prof. WANG Meng and Prof. MA Ding from Peking University, reported a advance in the indentification, separation, and catalytic transformation of real-life plastic waste mixtures.The researchers utilized an innovative solid-state NMR method—1H-13C Frequency Switched Lee Goldburg Heteronuclear Correlation (FSLG-HETCOR) NMR. By optimizing key parameters such as spinning rate, contact time, and homonuclear decoupling field strength, and using 13C-labeled tyrosine hydrochloride as a reference, the researchers obtained high-resolution “fingerprint” spectra of individual plastic components from an eight-plastic mixture containing PS (polystyrene), PLA (polylactic acid), PU (polyurethane), PC (polycarbonate), PVC (polyvinyl chloride), PET (polyethylene terephthalate), PE (polyethylene), and PP (polypropylene).The method obtained spectra with high signal intensity and good resolution in the indirect dimension, allowing for precise identification of various functional groups in the plastic mixture and enabling real-time tracking of their chemical evolution.Furthermore, the researchers demonstrated the feasibility, effectiveness, and universality of this method by monitoring the full catalytic separation and transformation of real-life plastic waste mixtures. Their NMR-based analysis enabled the mapping of each step in the conversion process—from complex mixtures to multiple high-value chemicals products.By identifying characteristic functional group signals in plastic waste mixtures, the researchers laid a solid foundation for their effective separation and transformation. This work paves the way for integrating existing transformation processes into a unified framework, providing technical support for scalable industrial solutions to global plastic pollution."Solid-state NMR provides a powerful way to identify individual components in plastic waste mixtures. It acts as a 'guiding eye' for the separation and catalytic transformation processes, laying the technological foundation for real-life solutions to plastic pollution," said Prof. XU. -
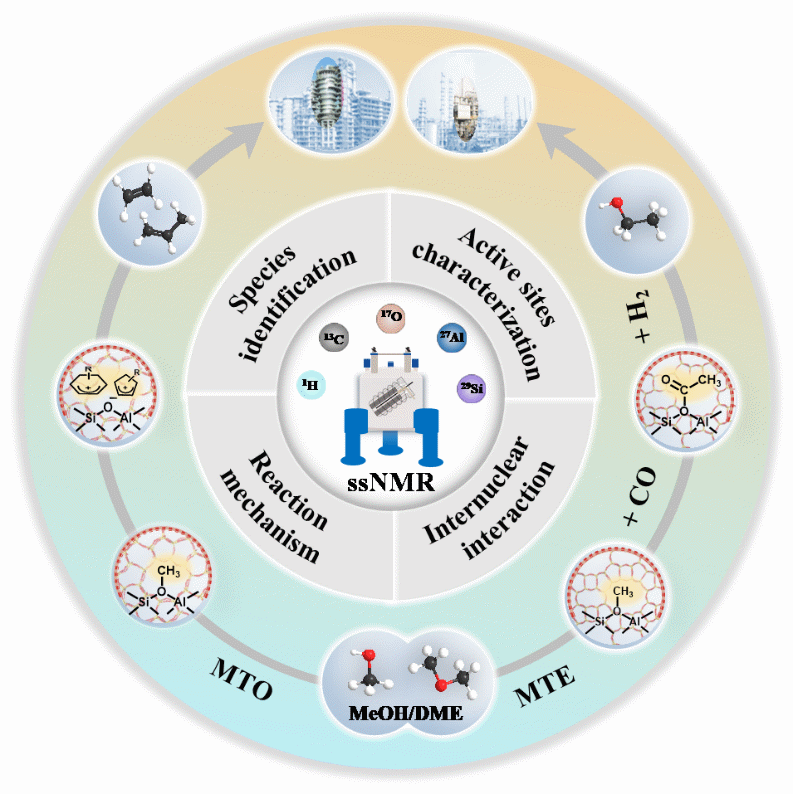 06 25, 2025Bridging Molecular Mechanisms and Industrial Processes of Zeolite-catalyzed Methanol Conversion Using Advanced Solid-state NMR SpectroscopyA research group led by Prof. XU Shutao, Prof. WEI Yingxu, and Prof. LIU Zhongmin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) provides a comprehensive overview of the application of solid-state nuclear magnetic resonance (ssNMR) spectroscopy in methanol to olefin (MTO) and dimethyl ether (DME) carbonylation reaction. DME carbonylation is a key step in the methanol to ethanol conversion process.With traditional petroleum-based routes facing increasing constraints, the conversion of C1 platform molecules, particularly methanol, has emerged as a crucial alternative for producing fundamental chemicals via non-petroleum pathways.Schematic depiction of the application of ssNMR spectroscopy during methanol conversion to olefins and ethanol process (Image by NIU Jing and DING Xinzhi)In a review article published in Chemical Society Reviews, a research group led by Prof. XU Shutao, Prof. WEI Yingxu, and Prof. LIU Zhongmin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) provides a comprehensive overview of the application of solid-state nuclear magnetic resonance (ssNMR) spectroscopy in methanol to olefin (MTO) and dimethyl ether (DME) carbonylation reaction. DME carbonylation is a key step in the methanol to ethanol conversion process.Researchers highlighted the pivotal role of ssNMRspectroscopy in advancing the understanding of methanol conversion mechanisms. It has enabled researchers to identify key intermediates, unravel dynamic catalytic mechanisms, probe host guest interactions, analyze diffusion behavior, and explore the unique role of water in these complex reactions.In situ ssNMR spectroscopy enables dynamic observation of catalytic processes under reaction conditions, offering valuable insights into the behavior of active catalysts. Additionally, two-dimensional ssNMR has revealed molecular and atomic level host guest interactions, deepening understanding of reaction pathways and catalyst interface properties.Despite these advances, researchers pointed out that a major challenge remains in bridging fundamental studies with industrial-scale MTO/DMTO technologies. They emphasized the need to validate laboratory-observed phenomena under industrial reactor conditions to ensure practical relevance. Continuous feedback from industrial applications is essential for refining theoretical models, optimizing catalyst design, and improving catalytic performance, which in turn promotes technological innovation and industrial upgrading."This review aims to bridge fundamental understandings of reaction mechanisms with practical applications," said Prof. XU. "Such insights are crucial for rational catalysts design, optimization of catalytic performance, and the improvement of industrial processes."
06 25, 2025Bridging Molecular Mechanisms and Industrial Processes of Zeolite-catalyzed Methanol Conversion Using Advanced Solid-state NMR SpectroscopyA research group led by Prof. XU Shutao, Prof. WEI Yingxu, and Prof. LIU Zhongmin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) provides a comprehensive overview of the application of solid-state nuclear magnetic resonance (ssNMR) spectroscopy in methanol to olefin (MTO) and dimethyl ether (DME) carbonylation reaction. DME carbonylation is a key step in the methanol to ethanol conversion process.With traditional petroleum-based routes facing increasing constraints, the conversion of C1 platform molecules, particularly methanol, has emerged as a crucial alternative for producing fundamental chemicals via non-petroleum pathways.Schematic depiction of the application of ssNMR spectroscopy during methanol conversion to olefins and ethanol process (Image by NIU Jing and DING Xinzhi)In a review article published in Chemical Society Reviews, a research group led by Prof. XU Shutao, Prof. WEI Yingxu, and Prof. LIU Zhongmin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) provides a comprehensive overview of the application of solid-state nuclear magnetic resonance (ssNMR) spectroscopy in methanol to olefin (MTO) and dimethyl ether (DME) carbonylation reaction. DME carbonylation is a key step in the methanol to ethanol conversion process.Researchers highlighted the pivotal role of ssNMRspectroscopy in advancing the understanding of methanol conversion mechanisms. It has enabled researchers to identify key intermediates, unravel dynamic catalytic mechanisms, probe host guest interactions, analyze diffusion behavior, and explore the unique role of water in these complex reactions.In situ ssNMR spectroscopy enables dynamic observation of catalytic processes under reaction conditions, offering valuable insights into the behavior of active catalysts. Additionally, two-dimensional ssNMR has revealed molecular and atomic level host guest interactions, deepening understanding of reaction pathways and catalyst interface properties.Despite these advances, researchers pointed out that a major challenge remains in bridging fundamental studies with industrial-scale MTO/DMTO technologies. They emphasized the need to validate laboratory-observed phenomena under industrial reactor conditions to ensure practical relevance. Continuous feedback from industrial applications is essential for refining theoretical models, optimizing catalyst design, and improving catalytic performance, which in turn promotes technological innovation and industrial upgrading."This review aims to bridge fundamental understandings of reaction mechanisms with practical applications," said Prof. XU. "Such insights are crucial for rational catalysts design, optimization of catalytic performance, and the improvement of industrial processes."