Research News
-
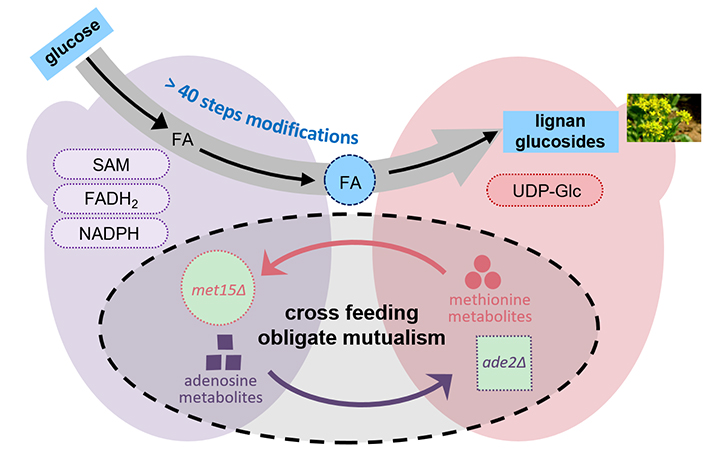 03 21, 2025Researchers Achieve De Novo Biosynthesis of Plant Lignans using Synthetic Yeast ConsortiaResearchres achieved the biosynthesis of the antiviral ingredient lignan glycoside in yeast Saccharomyces cerevisiae.Lignans are low molecular weight polyphenolic compounds with important clinical value, including antitumor and antiviral properties. However, their low amounts in medicinal plants and complex structures make sustainable production through plant extraction and chemical synthesis challenging, limiting their availability to meet market demand.A research group led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. ZHANG Lei and Prof. CHEN Wansheng from the Naval Medical University, has achieved the biosynthesis of the antiviral ingredient lignan glycoside in yeast Saccharomyces cerevisiae. The study was published in Nature Chemical BiologyDe novo biosynthesis of plant lignans by synthetic yeast consortia (Image by CHEN Ruibing)Researchers constructed a synthetic yeast consortium inspired by plant metabolism. By simulating the multicellular synthesis mechanism in plants, they addressed challenges related to side reactions and metabolic network promiscuity.Researchers designed two auxotrophic yeast strains (met15Δ and ade2Δ) to form a mutualistic relationship, cross-feeding metabolites while dividing the biosynthetic pathway into upstream and downstream. This enabled the de novo synthesis of lariciresinol diglucoside through over 40 enzymatic reactions."Our work demonstrates that Saccharomyces cerevisiae auxotrophic strains spontaneously establish a mutualistic community for the heterologous synthesis of complex active ingredients in traditional Chinese medicine," said Prof. ZHOU. "And this strategy is expected to be extended to the design of other stable cooperative yeast cell systems to accomplish complex bioengineering tasks," Prof. ZHOU added.
03 21, 2025Researchers Achieve De Novo Biosynthesis of Plant Lignans using Synthetic Yeast ConsortiaResearchres achieved the biosynthesis of the antiviral ingredient lignan glycoside in yeast Saccharomyces cerevisiae.Lignans are low molecular weight polyphenolic compounds with important clinical value, including antitumor and antiviral properties. However, their low amounts in medicinal plants and complex structures make sustainable production through plant extraction and chemical synthesis challenging, limiting their availability to meet market demand.A research group led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. ZHANG Lei and Prof. CHEN Wansheng from the Naval Medical University, has achieved the biosynthesis of the antiviral ingredient lignan glycoside in yeast Saccharomyces cerevisiae. The study was published in Nature Chemical BiologyDe novo biosynthesis of plant lignans by synthetic yeast consortia (Image by CHEN Ruibing)Researchers constructed a synthetic yeast consortium inspired by plant metabolism. By simulating the multicellular synthesis mechanism in plants, they addressed challenges related to side reactions and metabolic network promiscuity.Researchers designed two auxotrophic yeast strains (met15Δ and ade2Δ) to form a mutualistic relationship, cross-feeding metabolites while dividing the biosynthetic pathway into upstream and downstream. This enabled the de novo synthesis of lariciresinol diglucoside through over 40 enzymatic reactions."Our work demonstrates that Saccharomyces cerevisiae auxotrophic strains spontaneously establish a mutualistic community for the heterologous synthesis of complex active ingredients in traditional Chinese medicine," said Prof. ZHOU. "And this strategy is expected to be extended to the design of other stable cooperative yeast cell systems to accomplish complex bioengineering tasks," Prof. ZHOU added. -
 03 13, 2025Novel Solid-state Electrolyte Developed to Enhance Performance of All-solid-state Lithium-ion BatteriesResearchers developed a high ionic conductivity sulfide-based solid electrolytes, enhancing the performance of all-solid-state lithium-ion batteries.All-solid-state lithium-ion batteries (ASSBs) have garnered attention for their high energy density and superior safety. However, their commercialization remains challenging due to the lack of solid-state electrolytes (SSEs) with both high ionic conductivity and stable interfaces. To date, only a few SSEs have achieved ionic conductivities exceeding 10 mS/cm at room temperature.Recently, a research team led by Prof. WU Zhong-Shuai and Assoc. Prof. SHI Haodong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a high ionic conductivity sulfide-based solid electrolytes, enhancing the performance of ASSBs. Their study was published in ACS Energy Letters.A superionic Li20/3(GeSiSb)1/3S5I conductor is developed for all-solid-state power batteries (Image by MA Yuxin and SHI Haodong)Researchers developed the novel electrolyte, Li20/3(GeSiSb)1/3S5I (LGSSSI), by employing a multi-cation (Ge, Si, Sb) doping and substitution strategy. The electrolyte exhibited exceptional ionic conductivity, attributed to its low lithium-ion migration activation energy (0.17 eV). After cold pressing, LGSSSI achieved an ionic conductivity of 12.7 mS/cm, which further increased to 32.2 mS/cm after hot pressing.Intergraed with LGSSSI, ASSBs achieved high cycling stability under ultra-high cathode mass loading (100 mg/cm2) and across a wide temperature range (-20°C to 60°C). Additionally, LGSSSI demonstrated excellent interfacial compatibility with various cathode and anode materials, including LiNi0.8Mn0.1Co0.1O2, LiCoO2 cathodes, lithium-indium alloy, and silicon-carbon composite anodes."Our study lays a foundation for achieving ASSBs with wide temperature adaptability, high cathode loading, and long cycle life," said Prof. WU.
03 13, 2025Novel Solid-state Electrolyte Developed to Enhance Performance of All-solid-state Lithium-ion BatteriesResearchers developed a high ionic conductivity sulfide-based solid electrolytes, enhancing the performance of all-solid-state lithium-ion batteries.All-solid-state lithium-ion batteries (ASSBs) have garnered attention for their high energy density and superior safety. However, their commercialization remains challenging due to the lack of solid-state electrolytes (SSEs) with both high ionic conductivity and stable interfaces. To date, only a few SSEs have achieved ionic conductivities exceeding 10 mS/cm at room temperature.Recently, a research team led by Prof. WU Zhong-Shuai and Assoc. Prof. SHI Haodong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a high ionic conductivity sulfide-based solid electrolytes, enhancing the performance of ASSBs. Their study was published in ACS Energy Letters.A superionic Li20/3(GeSiSb)1/3S5I conductor is developed for all-solid-state power batteries (Image by MA Yuxin and SHI Haodong)Researchers developed the novel electrolyte, Li20/3(GeSiSb)1/3S5I (LGSSSI), by employing a multi-cation (Ge, Si, Sb) doping and substitution strategy. The electrolyte exhibited exceptional ionic conductivity, attributed to its low lithium-ion migration activation energy (0.17 eV). After cold pressing, LGSSSI achieved an ionic conductivity of 12.7 mS/cm, which further increased to 32.2 mS/cm after hot pressing.Intergraed with LGSSSI, ASSBs achieved high cycling stability under ultra-high cathode mass loading (100 mg/cm2) and across a wide temperature range (-20°C to 60°C). Additionally, LGSSSI demonstrated excellent interfacial compatibility with various cathode and anode materials, including LiNi0.8Mn0.1Co0.1O2, LiCoO2 cathodes, lithium-indium alloy, and silicon-carbon composite anodes."Our study lays a foundation for achieving ASSBs with wide temperature adaptability, high cathode loading, and long cycle life," said Prof. WU. -
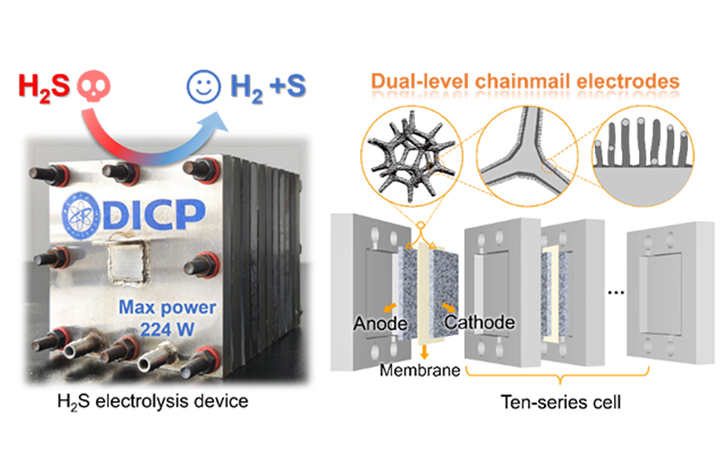 03 11, 2025Researchers Develop Chainmail Integrated-electrode for Highly Efficient Hydrogen Sulfide ElectrolysisResearchers developed a dual-level chainmail integrated-electrode that enables highly efficient hydrogen production via H2S electrolysis.Hydrogen sulfide (H2S), a toxic and corrosive byproduct of fossil fuel extraction, poses significant environmental and industrial challenges. While the conventional Claus process converts H2S into elemental sulfur, it fails to recover hydrogen gas, missing an opportunity for sustainable energy production.Electrocatalytic H2S decomposition offers a promising strategy to simultaneously eliminating pollutants and producing green hydrogen. However, the acidic nature of H2S deactivates non-precious metal catalysts and degrades electrode structures, making it difficult to achieve both high efficiency and long-term stability.Efficient H2S electrolysis to H2 production in the flow-cell device using chainmail integrated-electrode (Image by ZHANG Mo)In a study published in Angew. Chem. Int. Ed., a research group led by Prof. DENG Dehui and Assoc. Prof. CUI Xiaoju from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a dual-level chainmail integrated-electrode that enables highly efficient hydrogen production via H2S electrolysis.Researchers designed a graphene encapsulating nickel foam (Ni@NC foam) electrode with a dual-level chainmail structure, enhancing both catalytic activity and durability. This electrode achieved an industrial-scale current density exceeding 1 A/cm2 at 1.12 V versus the reversible hydrogen electrode, which was five times higher than commercial nickel foam electrodes. Moreover, the Ni@NC foam electrode remained stable for over 300 hours, demonstrating a lifespan at least ten times longer than commercial nickel foam electrodes.In a simulated natural gas desulfurization test, the chainmail integrated-electrode completely oxidized and removed 20% H2S at the anode, producing sulfur powder simultaneously. Meanwhile, high-purity hydrogen was collected at the cathode. Compared with conventional water electrolysis, the system reduced energy consumption by 43% at the current density of 200 mA/cm², offering a more sustainable approach to hydrogen production."Our study provides an efficient, low-energy solution for natural gas purification and opens up the potential of converting H2S into valuable hydrogen fuel for industrial applications," said Prof. DENG.
03 11, 2025Researchers Develop Chainmail Integrated-electrode for Highly Efficient Hydrogen Sulfide ElectrolysisResearchers developed a dual-level chainmail integrated-electrode that enables highly efficient hydrogen production via H2S electrolysis.Hydrogen sulfide (H2S), a toxic and corrosive byproduct of fossil fuel extraction, poses significant environmental and industrial challenges. While the conventional Claus process converts H2S into elemental sulfur, it fails to recover hydrogen gas, missing an opportunity for sustainable energy production.Electrocatalytic H2S decomposition offers a promising strategy to simultaneously eliminating pollutants and producing green hydrogen. However, the acidic nature of H2S deactivates non-precious metal catalysts and degrades electrode structures, making it difficult to achieve both high efficiency and long-term stability.Efficient H2S electrolysis to H2 production in the flow-cell device using chainmail integrated-electrode (Image by ZHANG Mo)In a study published in Angew. Chem. Int. Ed., a research group led by Prof. DENG Dehui and Assoc. Prof. CUI Xiaoju from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a dual-level chainmail integrated-electrode that enables highly efficient hydrogen production via H2S electrolysis.Researchers designed a graphene encapsulating nickel foam (Ni@NC foam) electrode with a dual-level chainmail structure, enhancing both catalytic activity and durability. This electrode achieved an industrial-scale current density exceeding 1 A/cm2 at 1.12 V versus the reversible hydrogen electrode, which was five times higher than commercial nickel foam electrodes. Moreover, the Ni@NC foam electrode remained stable for over 300 hours, demonstrating a lifespan at least ten times longer than commercial nickel foam electrodes.In a simulated natural gas desulfurization test, the chainmail integrated-electrode completely oxidized and removed 20% H2S at the anode, producing sulfur powder simultaneously. Meanwhile, high-purity hydrogen was collected at the cathode. Compared with conventional water electrolysis, the system reduced energy consumption by 43% at the current density of 200 mA/cm², offering a more sustainable approach to hydrogen production."Our study provides an efficient, low-energy solution for natural gas purification and opens up the potential of converting H2S into valuable hydrogen fuel for industrial applications," said Prof. DENG. -
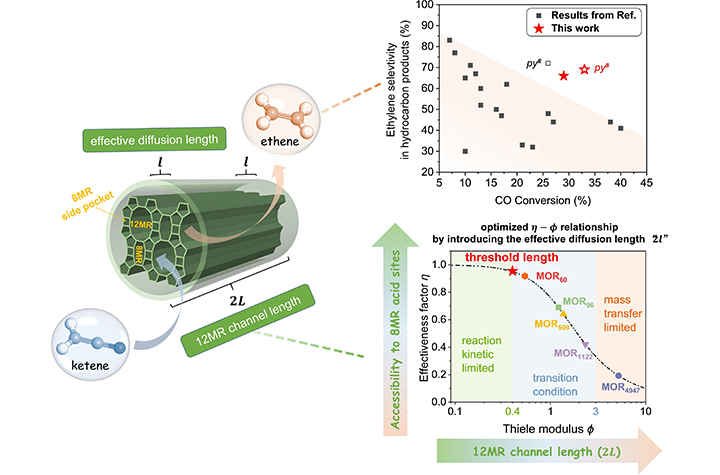 03 10, 2025Researchers Reveal the Role of Zeolite Acid Site Accessibility in Syngas ConversionResearchers revealed how the accessibility of zeolite acid site plays a crucial role in determining syngas conversion performance.Zeolites and zeotypes are widely used in the energy and chemical industries due to their unique pore structures and excellent shape-selective catalytic properties. However, these inherent advantages also lead to diffusion limitations, preventing guest molecules from effectively accessing internal active sites and thereby hindering catalytic efficiency.Schematic illustration of structure–mass transfer–activity relationships (Image by WANG Haodi)In a study published in Angewandte Chemie International Edition (VIP paper), a research group led by Prof. JIAO Feng and Prof. PAN Xiulian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed how the accessibility of zeolite acid site plays a crucial role in determining syngas conversion performance.Using mordenite (MOR) zeolite as a model catalyst, researchers investigated its unique pore structure, where acid sites within the 8-membered ring (8MR) side pockets serve as active sites for syngas-to-ethylene via OXZEO, while the 12-membered ring (12MR) channels act as molecular transport pathways.By systematically analyzing the mass transfer effects of MOR catalysts with varying 12MR channel lengths (2L), the researchers established a quantitative relationship between active sites accessibility and catalytic performance.Moreover, researchers identified 60 nm as the critical threshold for the 12MR channel length, where the reaction approached kinetic limitation. Using this property, they optimized the ZnAlOx-MOR bifunctional catalyst, achieving a CO conversion of 33% and an ethylene selectivity of 69%."Our study provides new insights into mass transfer mechanisms inside zeolites and offers a framework for designing high-performance zeolite-based catalysts," said Prof. JIAO.
03 10, 2025Researchers Reveal the Role of Zeolite Acid Site Accessibility in Syngas ConversionResearchers revealed how the accessibility of zeolite acid site plays a crucial role in determining syngas conversion performance.Zeolites and zeotypes are widely used in the energy and chemical industries due to their unique pore structures and excellent shape-selective catalytic properties. However, these inherent advantages also lead to diffusion limitations, preventing guest molecules from effectively accessing internal active sites and thereby hindering catalytic efficiency.Schematic illustration of structure–mass transfer–activity relationships (Image by WANG Haodi)In a study published in Angewandte Chemie International Edition (VIP paper), a research group led by Prof. JIAO Feng and Prof. PAN Xiulian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed how the accessibility of zeolite acid site plays a crucial role in determining syngas conversion performance.Using mordenite (MOR) zeolite as a model catalyst, researchers investigated its unique pore structure, where acid sites within the 8-membered ring (8MR) side pockets serve as active sites for syngas-to-ethylene via OXZEO, while the 12-membered ring (12MR) channels act as molecular transport pathways.By systematically analyzing the mass transfer effects of MOR catalysts with varying 12MR channel lengths (2L), the researchers established a quantitative relationship between active sites accessibility and catalytic performance.Moreover, researchers identified 60 nm as the critical threshold for the 12MR channel length, where the reaction approached kinetic limitation. Using this property, they optimized the ZnAlOx-MOR bifunctional catalyst, achieving a CO conversion of 33% and an ethylene selectivity of 69%."Our study provides new insights into mass transfer mechanisms inside zeolites and offers a framework for designing high-performance zeolite-based catalysts," said Prof. JIAO. -
 02 21, 2025DNL-17: Novel Small-pore Aluminophosphate Molecular SieveResearchers synthesized a novel small-pore AlPO MS, named DNL-17, using a flexible diquaternary ammonium compound as organic structure-directing agent (OSDA).Aluminophosphate (AlPO) molecular sieves (MSs) are crystalline microporous materials made from alternating PO₄ tetrahedra and AlO₄ tetrahedra, forming ordered channel systems and cage-like structures.Small-pore AlPO MSs with three-dimensional (3D) channel systems are particularly promising for selective adsorption and energy storage. However, it remains a challenge to synthesize and determine their crystallographic structures.In a study published in Journal of the American Chemical Society, a research group led by Prof. GUO Peng and Prof. LIU Zhongmin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) synthesized a novel small-pore AlPO MS, named DNL-17, using a flexible diquaternary ammonium compound as organic structure-directing agent (OSDA).Design, synthesis, and structural analysis of DNL-17 (Image by NIE Chenyang)Researchers used cutting-edge 3D electron diffraction (ED) technology to directly determine the complex crystallographic structure of DNL-17. This new member of the ABC-6 family features 3D 8 * 8 * 8-ring pores and a framework structure containingfour characteristic cages (d6r, can, eri, and cha), with a distinct 24-layer stacking sequence along the c axis (AABAACAABBCBBABBCCACCBCC).In addition, researchers identified a unique structure-directing effect in which the flexible OSDAs adopt various conformations to stabilize different cages during crystallization. They also demonstrated that DNL-17 shows promise for selective adsorption in the separation of n-butane and isobutane."This study demonstrates that OSDAs can construct novel AlPO MSs through different conformations, paving the way for the design and synthesis of new molecular sieves," said Prof. GUO.
02 21, 2025DNL-17: Novel Small-pore Aluminophosphate Molecular SieveResearchers synthesized a novel small-pore AlPO MS, named DNL-17, using a flexible diquaternary ammonium compound as organic structure-directing agent (OSDA).Aluminophosphate (AlPO) molecular sieves (MSs) are crystalline microporous materials made from alternating PO₄ tetrahedra and AlO₄ tetrahedra, forming ordered channel systems and cage-like structures.Small-pore AlPO MSs with three-dimensional (3D) channel systems are particularly promising for selective adsorption and energy storage. However, it remains a challenge to synthesize and determine their crystallographic structures.In a study published in Journal of the American Chemical Society, a research group led by Prof. GUO Peng and Prof. LIU Zhongmin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) synthesized a novel small-pore AlPO MS, named DNL-17, using a flexible diquaternary ammonium compound as organic structure-directing agent (OSDA).Design, synthesis, and structural analysis of DNL-17 (Image by NIE Chenyang)Researchers used cutting-edge 3D electron diffraction (ED) technology to directly determine the complex crystallographic structure of DNL-17. This new member of the ABC-6 family features 3D 8 * 8 * 8-ring pores and a framework structure containingfour characteristic cages (d6r, can, eri, and cha), with a distinct 24-layer stacking sequence along the c axis (AABAACAABBCBBABBCCACCBCC).In addition, researchers identified a unique structure-directing effect in which the flexible OSDAs adopt various conformations to stabilize different cages during crystallization. They also demonstrated that DNL-17 shows promise for selective adsorption in the separation of n-butane and isobutane."This study demonstrates that OSDAs can construct novel AlPO MSs through different conformations, paving the way for the design and synthesis of new molecular sieves," said Prof. GUO. -
 02 20, 2025
02 20, 2025Novel Bifacial Linker Developed to Prevent Heterointerfacial Delamination in Flexible Perovskite Solar Cells
Researchers developed a novel bifacial linker to prevent heterointerfacial delamination in flexible perovskite solar cells.Flexible perovskite solar cells (F-PSCs) have attracted attention for their potential in diverse applications. However, their commercialization is hindered by challenges related to low mechanical flexibility, which leads to poor adhesion between the perovskite absorber layer and the flexible substrate.A recent study published in Advanced Materials offers a promising solution to this problem with the development of a novel bifacial linker designed to prevent heterointerfacial delamination in F-PSCs. This study was conducted by Prof. YANG Dong and Prof. LIU Shengzhong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS).Schematic illustration demonstrating the experimental validation of robust heterointerfacial adhesion between perovskite and SnO2 with the addition of the bifacial linker BnBF3K (Image by YANG Shaoan and ZHU Xuejie)Researchers introduced a novel bifacial linker, potassium benzyl(trifluoro)borate (BnBF3K), to enhance the adhesion at the SnO2/perovskite interface, which effectively addreses the delamination issue in F-PSCs. By optimizing heterointerfacial delamination, minimizing buried defects in the perovskite, reducing SnO2 surface defects, and improving physical contact between the perovskite and the SnO2-coated substrate, they were able to significantly enhance the performance of the solar cells.Further analysis confirmed the crucial role of the bifacial linker in boosting device performance. The unique properties of BnBF3K facilitated strong molecular interactions and robust adsorption, ensuring excellent adhesion between the perovskite and the SnO2 substrate. The strengthened mechanical interface provided a stable foundation for electrical contact, allowing efficient charge extraction and transport, even under mechanical deformation of the flexible device.As a result of the bifacial linker, the researchers achieved an efficiency of 21.82% (certified at 21.39%) for a flexible perovskite solar module with an area of 12.80 cm2. Moreover, the flexible modules demonstrated excellent mechanical flexibility, retaining 96.56% of their initial efficiency after 6,000 bending cycles, highlighting their potential for a wide range of practical applications."Our study not only improves the mechanical stability of flexible perovskite devices but also reduces buried surface defects and optimizes energy level alignment," said Prof. LIU.