Research News
-
 04 28, 2025Researchers Discover Accelerated Reaction Between Criegee Intermediates and Water via Roaming MechanismA research team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) led by Professors YANG Xueming, ZHANG Donghui, DONG Wenrui and FU Bina discovered accelerated reaction between Criegee Intermediates and water via roaming mechanismCriegee intermediates (CIs)—highly reactive species formed when ozone reacts with alkenes in the atmosphere—play a crucial role in generating hydroxyl radicals (the atmosphere's "cleansing agents") and aerosols that impact climate and air quality. The syn-CH3CHOO is particularly important among these intermediates, accounting for 25-79% of all CIs depending on the season.Until now, scientists believed that syn-CH3CHOO primarily disappeared through self-decomposition. However, a research team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), led by Professors YANG Xueming, ZHANG Donghui, DONG Wenrui and FU Bina, has uncovered a surprising new pathway: isyn-CH3CHOO's reaction with atmospheric water vapor is approximately 100 times faster than previously predicted by theoretical models. The study was published in Nature Chemistry."This fundamentally changes our understanding of atmospheric chemistry," said Prof. YANG.Using advanced laser techniques, the researchers experimentally measured the reaction rate between syn-CH3CHOO and water vapor, finding it approximately 100 times faster than expected. To uncover the reason behind this acceleration, they constructed a high-accuracy full-dimensional (27D) potential energy surface using the fundamental invariant-neural network approach and performed full-dimensional dynamical calculations.The researchers revealed a "roaming mechanism" driven by strong dipole-dipole interactions between the molecules. Instead of following a direct minimum energy path, the molecules "roam" near each other, leading to a much higher probability of reaction. Under typical atmospheric conditions, this water-based reaction pathway is just as important as the self-decomposition process that has been thought to dominate.This discovery suggests that the conventional view that unimolecular decomposition predominantly governs the removal of syn-CH₃CHOO needs to be revised. Moreover, it highlights the critical importance of combining high-precision experimental data with advanced full-dimensional simulations to accurately predict complex chemical reactions.Beyond atmospheric science, the newly uncovered "roaming mechanism" could have wide-reaching implications, potentially affecting fields like combustion chemistry and astrochemistry, where long-range interactions play a major role in reaction dynamics.By revising the understanding of key atmospheric processes in this study, scientists may develop more accurate models of climate change and air quality, demonstrating how discoveries on the molecular level can have profound global impacts.Scientists discover accelerated reaction between Criegee Intermediates and water via roaming mechanism (Image by DICP)
04 28, 2025Researchers Discover Accelerated Reaction Between Criegee Intermediates and Water via Roaming MechanismA research team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) led by Professors YANG Xueming, ZHANG Donghui, DONG Wenrui and FU Bina discovered accelerated reaction between Criegee Intermediates and water via roaming mechanismCriegee intermediates (CIs)—highly reactive species formed when ozone reacts with alkenes in the atmosphere—play a crucial role in generating hydroxyl radicals (the atmosphere's "cleansing agents") and aerosols that impact climate and air quality. The syn-CH3CHOO is particularly important among these intermediates, accounting for 25-79% of all CIs depending on the season.Until now, scientists believed that syn-CH3CHOO primarily disappeared through self-decomposition. However, a research team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), led by Professors YANG Xueming, ZHANG Donghui, DONG Wenrui and FU Bina, has uncovered a surprising new pathway: isyn-CH3CHOO's reaction with atmospheric water vapor is approximately 100 times faster than previously predicted by theoretical models. The study was published in Nature Chemistry."This fundamentally changes our understanding of atmospheric chemistry," said Prof. YANG.Using advanced laser techniques, the researchers experimentally measured the reaction rate between syn-CH3CHOO and water vapor, finding it approximately 100 times faster than expected. To uncover the reason behind this acceleration, they constructed a high-accuracy full-dimensional (27D) potential energy surface using the fundamental invariant-neural network approach and performed full-dimensional dynamical calculations.The researchers revealed a "roaming mechanism" driven by strong dipole-dipole interactions between the molecules. Instead of following a direct minimum energy path, the molecules "roam" near each other, leading to a much higher probability of reaction. Under typical atmospheric conditions, this water-based reaction pathway is just as important as the self-decomposition process that has been thought to dominate.This discovery suggests that the conventional view that unimolecular decomposition predominantly governs the removal of syn-CH₃CHOO needs to be revised. Moreover, it highlights the critical importance of combining high-precision experimental data with advanced full-dimensional simulations to accurately predict complex chemical reactions.Beyond atmospheric science, the newly uncovered "roaming mechanism" could have wide-reaching implications, potentially affecting fields like combustion chemistry and astrochemistry, where long-range interactions play a major role in reaction dynamics.By revising the understanding of key atmospheric processes in this study, scientists may develop more accurate models of climate change and air quality, demonstrating how discoveries on the molecular level can have profound global impacts.Scientists discover accelerated reaction between Criegee Intermediates and water via roaming mechanism (Image by DICP) -
04 08, 2025Solid Oxide Electrolysis Cell Enables Super-dry Reforming of MethaneResearchers developed a novel process for the direct production of syngas via super-dry reforming of methane (CO2/CH4 ≥ 2).Dry reforming of methane (DRM) is a widely studied method for converting carbon dioxide (CO2) and methane (CH4) into syngas. Traditionally, this reaction operates with a CO2/CH4 feed ratio of 1. However, future feedstocks—such as CO2-rich natural gas—are expected to contain much higher concentrations of CO2, requiring costly separation processes to achieve the desired CH4.Recently, a joint research team led by Profs. WANG Guoxiong, XIAO Jianping, and BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a novel process for the direct production of syngas via super-dry reforming of methane (CO2/CH4 ≥ 2). Using high-temperature tandem electro-thermocatalysis based on solid oxide electrolysis cells (SOECs), their study—published in Nature Chemistry—offers a promising pathway for efficient and direct utilization of CO2-rich natural gas.Operating at 600 to 850 °C, SOECs are capable of converting CO2 and H2O into CO and H2. With high reaction rates, high energy efficiency, and low operating costs, SOECs have great potential for CO2 utilization, hydrogen production, and renewable energy storage. Considering the similar temperature range of SOECs and DRM, the researchers developed a novel process that coupled DRM, reverse water-gas shift (RWGS), and H2O electrolysis at the SOEC cathode.In this setup, the in situ electrochemical reduction of H2O byproduct generates H2 and O2- ions. These O2- ions then migrate through the electrolyte and are electrochemically oxidized to O2 at the anode under an applied potential. This process drives the RWGS equilibrium forward, enhancing CO2 conversion and H2 selectivity beyond conventional thermodynamic limitations.The researcher in suit exsolved Rh nanoparticles onto a CeO2-x support, creating high-density Ce3+-VO-Rhδ+ interfacial active sites. When operating at a CO2/CH4 ratio of 4, the system achieved CH4 conversion of 94.5% and CO2 conversion of 95.0%, with nearly 100% selectivity toward CO and H2. The apparent methane reducibility reached the theoretical maximum of 4.0.Further investigation revealed that Rhδ+ sites are primarily responsible for CH4 dissociation, while the Ce3+-VO-Rhδ+ interface—rich in oxygen vacancies—promotes CO2 adsorption, activation, and the RWGS reaction. This same interface also catalyzed electrochemical H2O reduction, boosting both CO2 conversion and H2 selectivity."Our study may open a new avenue for the direct utilization of CO2-rich natural gas and industrial tail gases using renewable energy," said Prof. WANG.
-
 04 03, 2025DICP Proposes Frontier Molecular Orbital Theory for Single-Atom Catalyst DesignResearchers innovatively introduced the Frontier Molecular Orbital (FMO) theory into SAC design.Single-atom catalysts (SACs), with their excellent metal atom utilization and unique physicochemical properties, hold promise for broad applications, especially in heterogeneous catalysis and energy conversions. Essentially, the activity and stability of SACs are governed by the pair of metal-adsorbate and metal-support interactions. However, the rationale of these interactions with their catalytic performance of SACs in nature and a unified theoretical model to describe both activity and stability remains elusive.To address this challenge, a collaborative team led by Prof. ZHANG Tao and Associate Prof. YANG Bing from the Dalian Institute of Chemical Physics (DICP) of Chinese Academy of Sciences (CAS), Prof. LU Junling and Prof. WU Xiaojun from the University of Science and Technology of China (USTC) innovatively introduced the Frontier Molecular Orbital (FMO) theory into SAC design. The study was published in Nature on April 2.In this work, the team constructed 34 palladium (Pd) SACs on 14 semiconductor supports. By adjusting support size and composition, they were able to precisely tuning the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) energy levels of the supports. The energy positions of LUMO and HOMO with particle size were experimentally determined by ultraviolet‒visible (UV‒Vis) spectroscopy and Mott–Schottky plots.Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) confirmed atomic dispersion of Pd on metal oxide particles (MOx). In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and X-ray photoelectron spectroscopy (XPS) further demonstrated enhanced Pd-MOx electronic interactions.In the reaction of semi-hydrogenation of acetylene, Pd SACs supported on nanoscale ZnO and CoOx exhibited a 20-fold activity enhancement over bulk oxide-supported counterparts while maintaining high selectivity.Notably, Pd1 SACs on 1.9 nm ZnO achieved a remarkable turnover frequency (TOF) of 25.6 min⁻¹ at 80 °C, surpassing all the Pd1 SACs reported in the literature. These catalysts also demonstrated exceptional stability over 100 hours without coke formation or metal aggregation.Correlation of the intrinsic activities with the properties of Pd1 discloses that the activities of Pd1/MOx SACs didn’t show a clear correlation with the Pd charge states. In contrast, their activities showed a linear scaling relationship with the LUMO positions of the n- and p-type oxide particle supports in Pd1/MOx.Theoretical calculations elucidated the underlying mechanism: reducing ZnO size elevates its LUMO level and widens the bandgap. The elevated LUMO of support reduces its energy gap with the HOMO of Pd1 atoms, which promotes Pd1–support orbital hybridizations for high stability.Meanwhile, the variation of Pd1–support orbital hybridizations further amend the LUMO of anchored Pd1 atoms to enhance Pd1–adsorbate interactions for high activity. These findings consist with the FMO theory.This work presents the first direct experimental substantiation of FMO theory in full view and provides a general descriptor for the design of a highly active and stable SAC for the first time. These findings open a new and operational approach for high-throughput screening of proper metal-support pairs to achieve high activity and stability, particularly powered by artificial intelligence.
04 03, 2025DICP Proposes Frontier Molecular Orbital Theory for Single-Atom Catalyst DesignResearchers innovatively introduced the Frontier Molecular Orbital (FMO) theory into SAC design.Single-atom catalysts (SACs), with their excellent metal atom utilization and unique physicochemical properties, hold promise for broad applications, especially in heterogeneous catalysis and energy conversions. Essentially, the activity and stability of SACs are governed by the pair of metal-adsorbate and metal-support interactions. However, the rationale of these interactions with their catalytic performance of SACs in nature and a unified theoretical model to describe both activity and stability remains elusive.To address this challenge, a collaborative team led by Prof. ZHANG Tao and Associate Prof. YANG Bing from the Dalian Institute of Chemical Physics (DICP) of Chinese Academy of Sciences (CAS), Prof. LU Junling and Prof. WU Xiaojun from the University of Science and Technology of China (USTC) innovatively introduced the Frontier Molecular Orbital (FMO) theory into SAC design. The study was published in Nature on April 2.In this work, the team constructed 34 palladium (Pd) SACs on 14 semiconductor supports. By adjusting support size and composition, they were able to precisely tuning the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) energy levels of the supports. The energy positions of LUMO and HOMO with particle size were experimentally determined by ultraviolet‒visible (UV‒Vis) spectroscopy and Mott–Schottky plots.Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) confirmed atomic dispersion of Pd on metal oxide particles (MOx). In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and X-ray photoelectron spectroscopy (XPS) further demonstrated enhanced Pd-MOx electronic interactions.In the reaction of semi-hydrogenation of acetylene, Pd SACs supported on nanoscale ZnO and CoOx exhibited a 20-fold activity enhancement over bulk oxide-supported counterparts while maintaining high selectivity.Notably, Pd1 SACs on 1.9 nm ZnO achieved a remarkable turnover frequency (TOF) of 25.6 min⁻¹ at 80 °C, surpassing all the Pd1 SACs reported in the literature. These catalysts also demonstrated exceptional stability over 100 hours without coke formation or metal aggregation.Correlation of the intrinsic activities with the properties of Pd1 discloses that the activities of Pd1/MOx SACs didn’t show a clear correlation with the Pd charge states. In contrast, their activities showed a linear scaling relationship with the LUMO positions of the n- and p-type oxide particle supports in Pd1/MOx.Theoretical calculations elucidated the underlying mechanism: reducing ZnO size elevates its LUMO level and widens the bandgap. The elevated LUMO of support reduces its energy gap with the HOMO of Pd1 atoms, which promotes Pd1–support orbital hybridizations for high stability.Meanwhile, the variation of Pd1–support orbital hybridizations further amend the LUMO of anchored Pd1 atoms to enhance Pd1–adsorbate interactions for high activity. These findings consist with the FMO theory.This work presents the first direct experimental substantiation of FMO theory in full view and provides a general descriptor for the design of a highly active and stable SAC for the first time. These findings open a new and operational approach for high-throughput screening of proper metal-support pairs to achieve high activity and stability, particularly powered by artificial intelligence. -
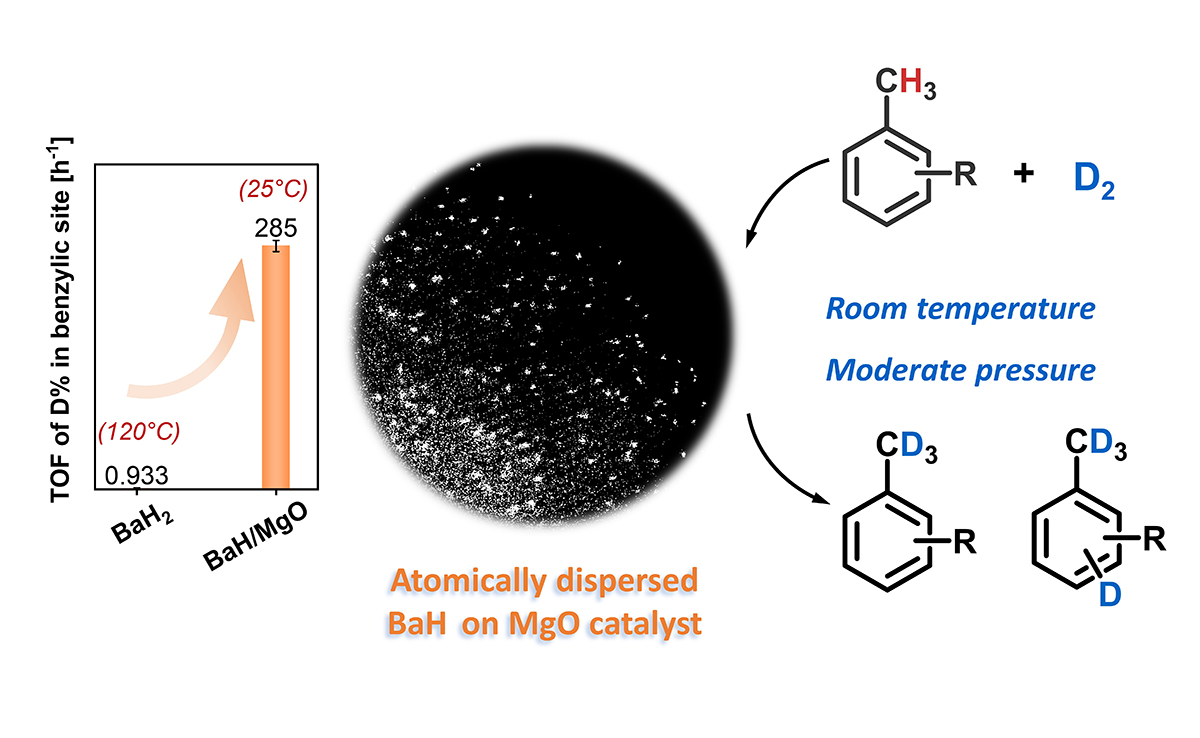 04 03, 2025Atomically Dispersed Barium Hydride Catalysts Developed for Deuteration of Nonactivated AlkylarenesResearchers developed atomically dispersed barium hydride catalysts for the synthesis of deuterated alkylarenes.Alkali and alkaline earth metal hydrides hold great promise for hydrogen storage and hydrogen-involved chemical transformations due to the unique properties of hydridic hydrogen (H‾). However, bulk hydrides often suffer from high lattice energy and limited exposure of active sites, hindering their catalytic performance.Recently, a research group led by Prof. GUO Jianping and Prof. CHEN Ping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. CHANG Fei from Yongjiang Laboratory and Prof. RAO Li from Central China Normal University, developed atomically dispersed barium hydride catalysts for the synthesis of deuterated alkylarenes. This study was published inNature Communications.Efficient deuteration of alkylarenes catalyzed by atomically-dispersed barium hydride materials (Image by CAI Yongli)Researchers synthesized atomically dispersed barium hydride catalysts on magnesium oxide (BaH/MgO) using a convenient impregnation-hydrogenation method. This (sub)nanostructured hydride material acts as an efficient, transition metal-free heterogeneous catalyst for hydrogen activation and hydrogen isotope exchange reactions across a range of nonactivated alkylarene substrates.The catalyst enabled regioselective deuteration of benzylic sites in toluene and its derivatives, achieving a high turnover frequency that surpasses both homogeneous and heterogeneous counterparts. Besides, it facilitated the deuteration of non-directional aromatics at room temperature with efficiency comparable to transition metal-catalyzed processes, which typically require elevated temperatures.Theoretical studies revealed that the surface barium hydride species play a multifaceted role, enabling deprotonation of sp3C-H bonds and nucleophilic attack on the sp2C atoms to yield regioselective products."This study offers an alternative synthetic route for the production of deuterium-labeled compounds and shows potential for further applications in tritium labeling of alkylarenes," said Prof. CHEN. "This new method for preparing (sub)nanostructured metal hydrides may open new avenues for their applications in diverse chemical processes," said Prof. GUO.
04 03, 2025Atomically Dispersed Barium Hydride Catalysts Developed for Deuteration of Nonactivated AlkylarenesResearchers developed atomically dispersed barium hydride catalysts for the synthesis of deuterated alkylarenes.Alkali and alkaline earth metal hydrides hold great promise for hydrogen storage and hydrogen-involved chemical transformations due to the unique properties of hydridic hydrogen (H‾). However, bulk hydrides often suffer from high lattice energy and limited exposure of active sites, hindering their catalytic performance.Recently, a research group led by Prof. GUO Jianping and Prof. CHEN Ping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. CHANG Fei from Yongjiang Laboratory and Prof. RAO Li from Central China Normal University, developed atomically dispersed barium hydride catalysts for the synthesis of deuterated alkylarenes. This study was published inNature Communications.Efficient deuteration of alkylarenes catalyzed by atomically-dispersed barium hydride materials (Image by CAI Yongli)Researchers synthesized atomically dispersed barium hydride catalysts on magnesium oxide (BaH/MgO) using a convenient impregnation-hydrogenation method. This (sub)nanostructured hydride material acts as an efficient, transition metal-free heterogeneous catalyst for hydrogen activation and hydrogen isotope exchange reactions across a range of nonactivated alkylarene substrates.The catalyst enabled regioselective deuteration of benzylic sites in toluene and its derivatives, achieving a high turnover frequency that surpasses both homogeneous and heterogeneous counterparts. Besides, it facilitated the deuteration of non-directional aromatics at room temperature with efficiency comparable to transition metal-catalyzed processes, which typically require elevated temperatures.Theoretical studies revealed that the surface barium hydride species play a multifaceted role, enabling deprotonation of sp3C-H bonds and nucleophilic attack on the sp2C atoms to yield regioselective products."This study offers an alternative synthetic route for the production of deuterium-labeled compounds and shows potential for further applications in tritium labeling of alkylarenes," said Prof. CHEN. "This new method for preparing (sub)nanostructured metal hydrides may open new avenues for their applications in diverse chemical processes," said Prof. GUO. -
 04 01, 2025With a Dash of Water and Sunlight: Researchers Turn Propane into Propylene Using Copper Single-Atom CatalystResearchers developed a water-catalyzed PDH reaction route using a copper single-atom catalyst (SAC) through photo-thermo catalysis, which enabled highly efficient propane-to-propylene conversion under mild conditions.Propane dehydrogenation reaction (PDH) is a highly endothermic reaction, typically requiring temperature above 600°C in conventional thermo-catalysis. However, elevated temperatures lead to significant energy consumption, catalyst sintering, and coke deposition. Overcoming these thermodynamic and kinetic challenges to achieve propane dehydrogenation under ambient conditions remains a major goal in catalysis.Recently, a research team led by Prof. ZHANG Tao and Prof. WANG Aiqin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. GAO Yi's team from the Shanghai Advanced Research Institute of CAS, developed a water-catalyzed PDH reaction route using a copper single-atom catalyst (SAC) through photo-thermo catalysis. This study, published in Nature Chemistry, enables highly efficient propane-to-propylene conversion under mild conditions.By using a Cu1/TiO2 SAC, researchers achieved PDH under near-ambient conditions in a water vapor atmosphere. The reaction temperature was reduced to just 50–80 °C in a continuous-flow fixed-bed reactor, achieving a maximum reaction rate of 1201 μmol gcat-1 h-1.The study revealed that Cu single atoms, water vapor, and light illumination all played essential roles in the propane-to-propylene conversion. Through photocatalytic water splitting on the Cu1/TiO2 SAC, hydrogen and hydroxyl species were generated. Hydroxyl radicals subsequently adsorbed on the catalyst surface, abstracting hydrogen atoms from propane to form propylene and water. Notably, water acted catalytically without being consumed. This mechanism fundamentally differs from traditional PDH and oxidative dehydrogenation of propane (ODHP).Furthermore, the researchers demonstrated that this strategy could be extended to the dehydrogenation of other light alkanes, including ethane and butane. The reaction could even be directly driven by sunlight using the Cu1/TiO2 SAC."Our study not only provides a novel pathway for PDH but also establishes a paradigm for conducting high-temperature reactions driven by solar energy," said Prof. LIU Xiao Yan, one of the corresponding authors of this study.<!--!doctype-->
04 01, 2025With a Dash of Water and Sunlight: Researchers Turn Propane into Propylene Using Copper Single-Atom CatalystResearchers developed a water-catalyzed PDH reaction route using a copper single-atom catalyst (SAC) through photo-thermo catalysis, which enabled highly efficient propane-to-propylene conversion under mild conditions.Propane dehydrogenation reaction (PDH) is a highly endothermic reaction, typically requiring temperature above 600°C in conventional thermo-catalysis. However, elevated temperatures lead to significant energy consumption, catalyst sintering, and coke deposition. Overcoming these thermodynamic and kinetic challenges to achieve propane dehydrogenation under ambient conditions remains a major goal in catalysis.Recently, a research team led by Prof. ZHANG Tao and Prof. WANG Aiqin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. GAO Yi's team from the Shanghai Advanced Research Institute of CAS, developed a water-catalyzed PDH reaction route using a copper single-atom catalyst (SAC) through photo-thermo catalysis. This study, published in Nature Chemistry, enables highly efficient propane-to-propylene conversion under mild conditions.By using a Cu1/TiO2 SAC, researchers achieved PDH under near-ambient conditions in a water vapor atmosphere. The reaction temperature was reduced to just 50–80 °C in a continuous-flow fixed-bed reactor, achieving a maximum reaction rate of 1201 μmol gcat-1 h-1.The study revealed that Cu single atoms, water vapor, and light illumination all played essential roles in the propane-to-propylene conversion. Through photocatalytic water splitting on the Cu1/TiO2 SAC, hydrogen and hydroxyl species were generated. Hydroxyl radicals subsequently adsorbed on the catalyst surface, abstracting hydrogen atoms from propane to form propylene and water. Notably, water acted catalytically without being consumed. This mechanism fundamentally differs from traditional PDH and oxidative dehydrogenation of propane (ODHP).Furthermore, the researchers demonstrated that this strategy could be extended to the dehydrogenation of other light alkanes, including ethane and butane. The reaction could even be directly driven by sunlight using the Cu1/TiO2 SAC."Our study not only provides a novel pathway for PDH but also establishes a paradigm for conducting high-temperature reactions driven by solar energy," said Prof. LIU Xiao Yan, one of the corresponding authors of this study.<!--!doctype--> -
 03 28, 2025Researchers Track Photogenerated Charge Transfer in ElectrolyteSequential imaging reveals reaction environment-induced interfacial electric field reversalPhotocatalysis involves three fundamental steps: light absorption, charge separation and transfer, and chemical reactions. These reactions occur at the solid-liquid interface where the complex charged environment influences reaction kinetics. Most research has focused on the charge transfer processes within solid catalysts.Understanding the interplay between surface charges and charge transfer at the catalyst-electrolyte interface is critical for advancing photocatalysis. However, directly measuring surface charges in electrolytes at the nanoscale has long been a challenge.Recently, Prof. FAN Fengtao and Prof. LI Can et al. from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) have developed an innovative approach to measure surface charges in liquid environments. This study was published in the Journal of the American Chemical Society.By using a charged probe to isolate electrostatic forces from long-range interactions, researchers successfully mapped the electric field distribution within the electrical double layer. This method enabled direct measurement of surface potential and photovoltage under realistic liquid conditions. Moreover, they uncovered a key phenomenon: Surface charges at the solid-liquid interface create an additional driving force, pulling photogenerated electrons to the surface and driving the charge transfer reaction.Surface charge-induced interface electric field reversal and comparative imaging in air vs. electrolyte environments (Image by LI Qian and CUI Junhao)Researchers quantitatively demonstrated how local surface potential in the electrolyte varies with pH, providing micro-nano scale observations. By relating the surface potential and reaction product flux, they demonstrated that the photocatalytic oxygen evolution reaction rate is controlled by the surface charge induced electric field. Additionally, researchers identified the optimal pH range for efficient spatial separation of photogenerated electrons and holes. They visualized the entire charge transfer process from the space charge region to the active reaction sites."This imaging framework offers a platform to directly measure surface potential and reaction current under operational conditions, providing idea into photocatalytic charge transfer kinetics at the nanoscale," said Prof. FAN. "It provides new avenues for designing efficient photocatalysts and optimizing reaction conditions," added Prof. FAN."Our findings provide valuable understandings into addressing the bottleneck issues of photocatalytic reactions," said LI Can.
03 28, 2025Researchers Track Photogenerated Charge Transfer in ElectrolyteSequential imaging reveals reaction environment-induced interfacial electric field reversalPhotocatalysis involves three fundamental steps: light absorption, charge separation and transfer, and chemical reactions. These reactions occur at the solid-liquid interface where the complex charged environment influences reaction kinetics. Most research has focused on the charge transfer processes within solid catalysts.Understanding the interplay between surface charges and charge transfer at the catalyst-electrolyte interface is critical for advancing photocatalysis. However, directly measuring surface charges in electrolytes at the nanoscale has long been a challenge.Recently, Prof. FAN Fengtao and Prof. LI Can et al. from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) have developed an innovative approach to measure surface charges in liquid environments. This study was published in the Journal of the American Chemical Society.By using a charged probe to isolate electrostatic forces from long-range interactions, researchers successfully mapped the electric field distribution within the electrical double layer. This method enabled direct measurement of surface potential and photovoltage under realistic liquid conditions. Moreover, they uncovered a key phenomenon: Surface charges at the solid-liquid interface create an additional driving force, pulling photogenerated electrons to the surface and driving the charge transfer reaction.Surface charge-induced interface electric field reversal and comparative imaging in air vs. electrolyte environments (Image by LI Qian and CUI Junhao)Researchers quantitatively demonstrated how local surface potential in the electrolyte varies with pH, providing micro-nano scale observations. By relating the surface potential and reaction product flux, they demonstrated that the photocatalytic oxygen evolution reaction rate is controlled by the surface charge induced electric field. Additionally, researchers identified the optimal pH range for efficient spatial separation of photogenerated electrons and holes. They visualized the entire charge transfer process from the space charge region to the active reaction sites."This imaging framework offers a platform to directly measure surface potential and reaction current under operational conditions, providing idea into photocatalytic charge transfer kinetics at the nanoscale," said Prof. FAN. "It provides new avenues for designing efficient photocatalysts and optimizing reaction conditions," added Prof. FAN."Our findings provide valuable understandings into addressing the bottleneck issues of photocatalytic reactions," said LI Can.