Directly converting methane (CH4) to high value-added multi-carbon (C2+) oxygenates, such as acetic acid (CH3COOH), under mild conditions presents a promising pathway for upgrading natural gas to transportable liquid chemicals.
Oxidative carbonylation of CH4 with carbonic oxide (CO) and oxygen (O2) to CH3COOH under mild conditions is an attractive and environmentally friendly route for CH4 utilization. However, this process involves complex reactions, including the activation of O2, efficient CH4 activation, and controllable C–C coupling. Therefore, it's a great challenge to achieve CH4, CO, and O2 to CH3COOH with both high catalytic activity and selectivity for the conversion of CH4 under mild conditions.
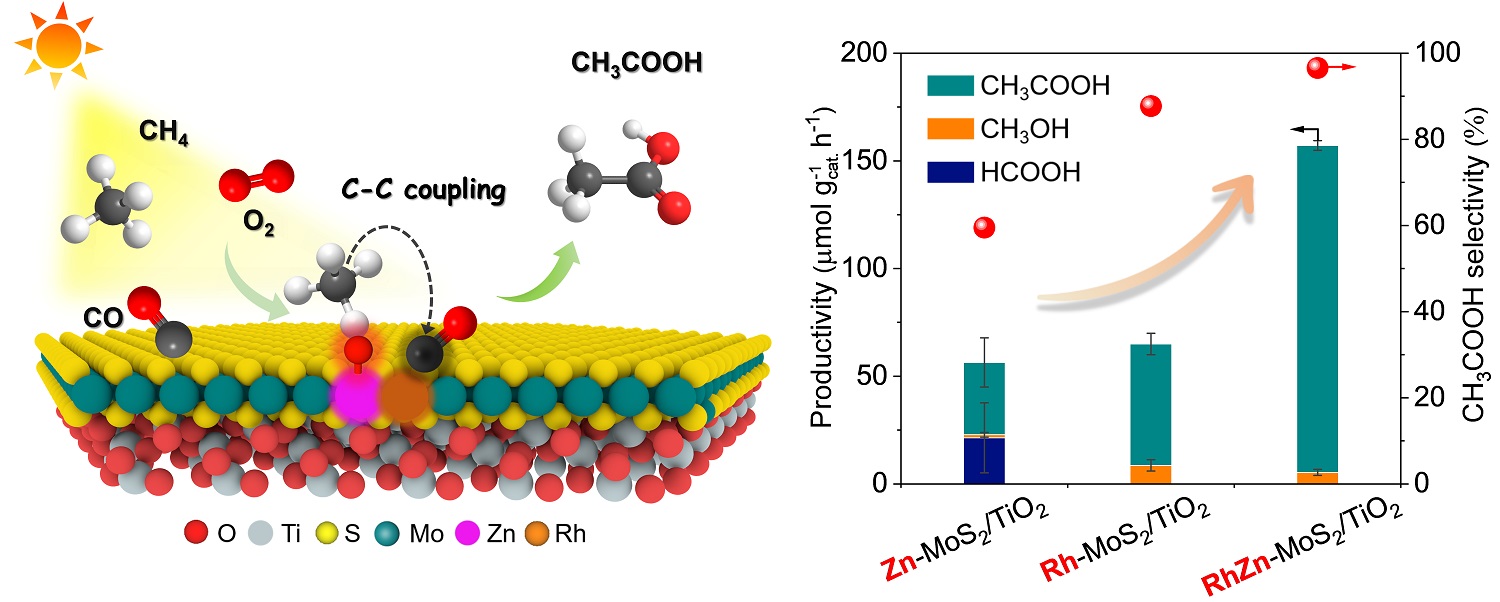
Schematic illustration of the photo-driven CH4 carbonylation with CO and O2 to CH3COOH over the RhZn-MoS2/TiO2 and the comparison of catalytic activity for different catalysts (Image by LI Yanan and LIU Huan)
In a study published in Nature Communications, a research group led by Prof. DENG Dehui, Assoc. Prof. CUI Xiaoju, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) have successfully achieved highly efficient photo-driven carbonylation of CH4 with CO and O2 to CH3COOH using a nano-heterostructure catalyst. This catalyst features Rh-Zn atomic-pair dual sites confined within a MoS2 lattice, integrated with TiO2 nanoparticles. This innovative catalyst enables a CH3COOH productivity of 152 μmol gcat.-1 h-1, and a turnover frequency of 62 h-1 with a high selectivity of 96.5%.
Moreover, the researchers revealed that the active OH species, produced from O2 photoreduction at the Zn site through proton-coupled electron transfer, promote CH4 dissociation to CH3 species. These CH3 species then easily couple with adsorbed CO on the adjacent Rh site, leading to highly selective CH3COOH formation. The Rh-Zn atomic-pair dual sites provide separated catalytic sites for C–H activation and C–C coupling, establishing a synergistic effect that overcomes the typical trade-off between activity and selectivity in CH4 carbonylation.
"Our study opens a new horizon to design efficient catalyst and provides a new pathway for photo-driven CH4 carbonylation to CH3COOH," said Prof. DENG.