The direct conversion of methane (CH4) into high value-added multi-carbon (C2+) oxygenates, such as acetic acid (CH3COOH), under mild conditions offers a promising pathway for upgrading natural gas into transportable liquid chemicals. However, achieving this transformation efficiently remains a major challenge due to the strong C-H bonds in methane, the difficulty of oxygen (O2) activation, and the low selectivity of C-C coupling reactions.
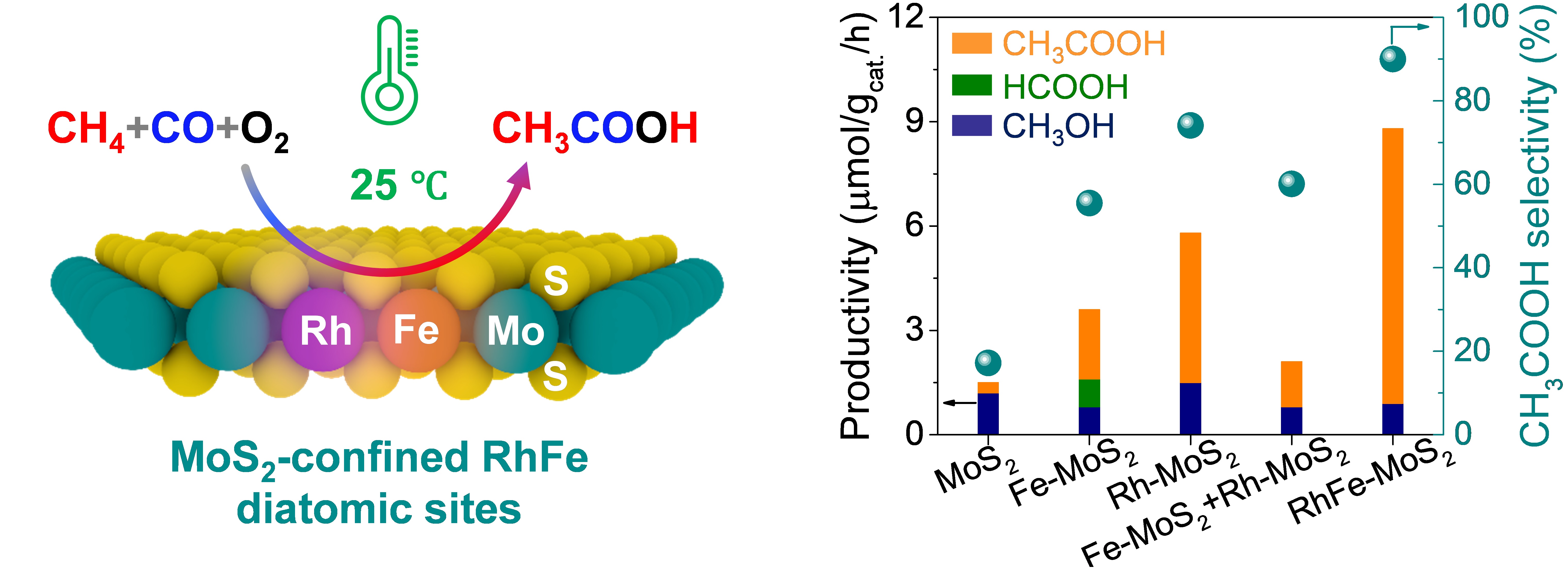
Schematic illustration of the CH4 carbonylation with CO and O2 to CH3COOH over the RhFe-MoS2 and the comparison of catalytic activity for different catalysts (Image by MAO Jun and LIU Huan)
In a study published in Journal of the American Chemical Society, a research group led by Prof. DENG Dehui, Assoc. Prof. CUI Xiaoju, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) have successfully achieved the direct carbonylation of CH4 with carbon monoxide (CO) and O2 into CH3COOH under mild conditions—including room temperature.
Using a novel MoS2-confined Rh-Fe dual-site catalyst, the researchers obtained an unprecedented CH3COOH selectivity of 90.3% and a productivity of 26.2 μmol gcat.–1 h–1 at just 25 °C, outperforming previously reported catalytic systems.
The researchers revealed that the confined Fe sites within MoS2 activate O2 to form highly reactive Fe=O species, which can dissociate CH4 into CH3 species even at room temperature. These CH3 species then couple with adsorbed CO on adjacent Rh sites to form the key CH3CO intermediate, leading to the formation of CH3COOH. The unique structure of Rh–Fe sites offers synergistic catalytic properties that effectively balance C–H activation and C–C coupling, addressing the trade-off between activity and selectivity in the carbonylation of CH4 to CH3COOH under mild conditions.
"Our study opens new avenues for designing efficient catalysts for the oxidative carbonylation of methane to acetic acid." said Prof. DENG.