Electrocatalytic nitric oxide reduction reaction (NORR) offers a promising route for sustainable ammonia (NH3) synthesis and for removing NO pollutants. However, achieving NH3 production from NO with ampere-level current density and long-term stability remains a challenge for industrial applications.
One major obstacle is the poor solubility of NO in water, combined with the undesirable hydrogen evolution reaction (HER), which limits the efficiency and durability of NH3 production on an industrial scale.
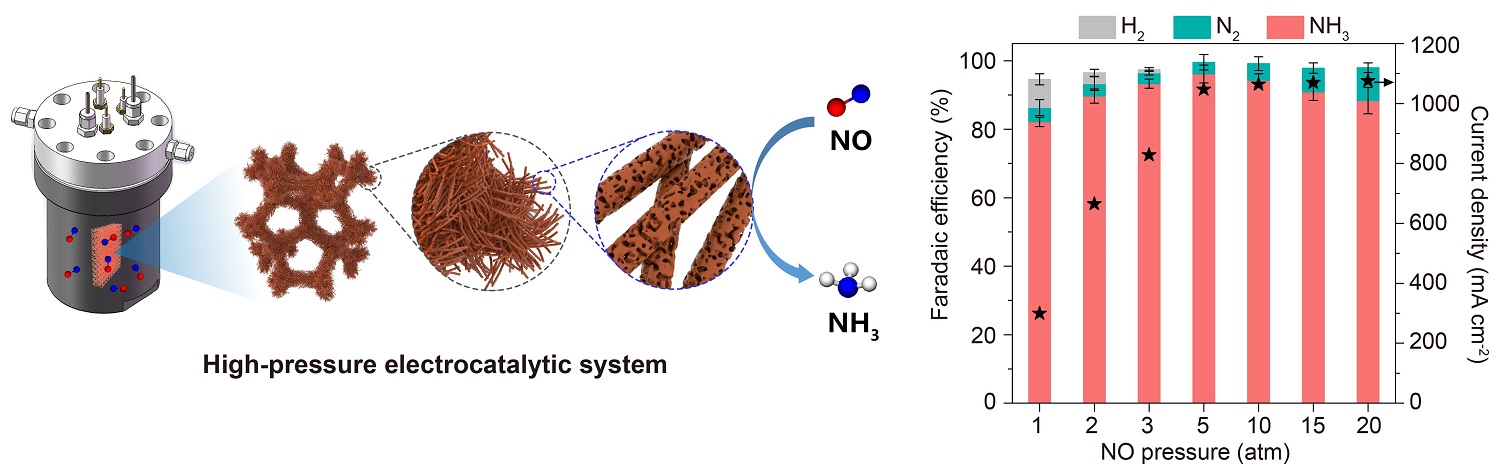
Schematic illustration of the high-pressure electrocatalytic system featuring a pressurized electrolyzer paired with a hierarchical porous Cu nanowire array (Cu NWA) monolithic electrode and the comparison of NORR activity under different NO pressure (Image by YANG Wenqiang and LIU Huan)
In a study published in Nature Communications, a research group led by Prof. DENG Dehui, Assoc. Prof. CUI Xiaoju, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a sustainable electrosynthesis method for NH3 from NO, achieving ampere-level current density in a pressurized electrolyzer.
Researchers used an in situ-grown hierarchical porous copper nanowire array (Cu NWA) monolithic electrode to regulate the kinetics and thermodynamics of the NORR. They achieved a NH3 partial current density of 1007 mA cm–2, a Faradaic efficiency of 96.1%, and an NH3 yield rate of 10.5 mmol h–1 cm–2, remaining stable at 1000 mA cm–2 for over 100 hours, demonstrating great potential for industrial-scale applications.
The high NORR performance was revealed to benefit from the in situ-formed hierarchical porous structure of the Cu NWA electrode. This structure maximized the exposure of Cu active sites, enhancing internal mass transfer. The high-pressure environment within the electrolyzer also enhanced NO solvation and external mass transfer, thereby promoting NO adsorption onto the Cu surface. Moreover, the high NO coverage destabilized the adsorbed NO and weakened hydrogen adsorption, facilitating efficient NO hydrogenation to NH3 while simultaneously suppressing the HER.
"Our study provides a new way for the industrial electrosynthesis of ammonia and the efficient catalytic conversion of inert small molecules," said Prof. DENG.