Ammonia (NH3), an important chemical for agriculture and industry, is traditionally produced through the energy-intensive Haber-Bosch process. This process converts nitrogen (N2) and hydrogen (H2) into NH3 at high temperatures (400−500 ℃) and pressures (10−30 MPa), consumes 1–2% of global energy and contributes about 1% of global CO2 emission.
A promising alternative is the electrocatalytic nitrate reduction reaction (NO3−RR), which is a renewable energy-driven process that uses nitrate (NO3−) from wastewater as the nitrogen source and water as the hydrogen source. This low-carbon route provides a sustainable solution for NH3 synthesis under mild conditions. However, its practical application has been limited by unsatisfactory electrocatalytic activity and poor long-term stability.
Recently, a research team led by Profs. GAO Dunfeng, WANG Guoxiong, and BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved high-rate and stable ammonia electrosynthesis from nitrate. Their findings, published in Nature Communications, introduced an amorphous/crystalline dual-phase Cu foam electrode with high performance.
High-rate ammonia electrosynthesis from nitrate using a stable amorphous/crystalline dual-phase Cu catalyst (Image by WANG Yi)
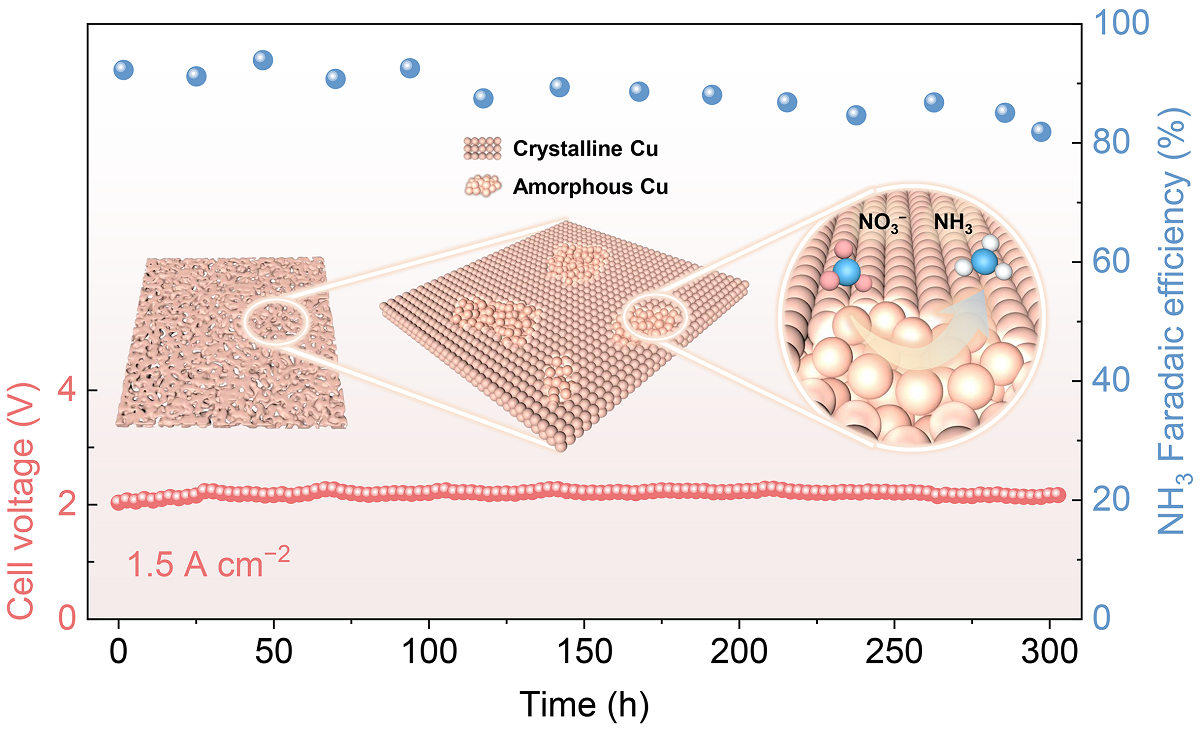
The researchers fabricated the electrode by thermal annealing commercial Cu foam in air, creating a unique dual-phase structure. With an alkaline membrane electrode assembly electrolyzer, the researchers achieved an NH3 partial current density of 3.33 A/cm2 and an ammonia formation rate of 15.5 mmol/h/cm2 at a cell voltage of just 2.6 V. Additionally, the electrode maintained stable NH3 production with a Faradaic efficiency of around 90% at an applied current density of 1.5 A/cm2 over 300 hours.
Furthermore, the researchers also identified that the stable amorphous Cu domains present during the reaction are key to outstanding catalytic performance. This integrated Cu foam electrode also performs better than conventional power electrodes, and the preparation protocols are facile and easy to scale up. A scale-up demonstration using a 100 cm2 electrode achieves an NH3 formation rate of up to 11.9 g/h at an applied current of 160 A.
"This work demonstrates the great promise of the integrated amorphous/crystalline dual-phase Cu foam electrode for high-rate and stable ammonia electrosynthesis from nitrate," said Prof. GAO.
"Our work also underscores the importance of stabilizing metastable amorphous structures for improving electrocatalytic reactivity and long-term stability," said Prof. WANG.