Research News
-
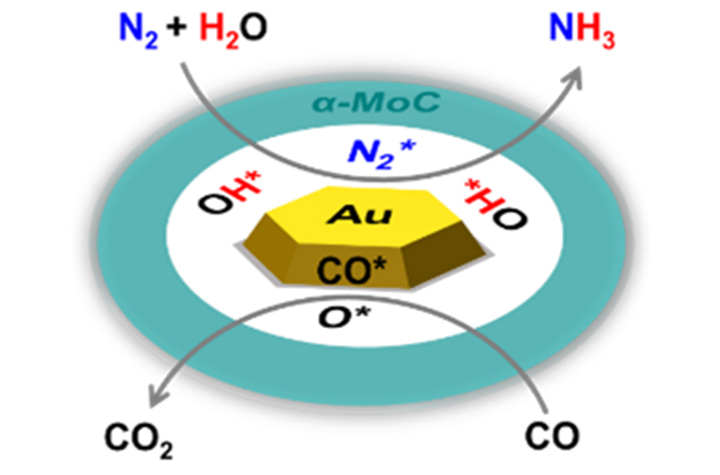 12 18, 2025Researchers Achieve Direct Ammonia Synthesis from Nitrogen and Water under Mild ConditionsA research team led by Prof. DENG Dehui, Prof. HUANG Rui, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved ammonia synthesis directly from nitrogen and water under mild conditions by introducing carbon monoxide into the reaction system to regulate overall reaction thermodynamics.The direct synthesis of ammonia (NH3) from nitrogen (N2) and water (H2O) under mild conditions presents a promising pathway for energy-efficient and sustainable NH3 production. However, thermocatalytic NH3 synthesis from N2 and H2O is limited by unfavorable reaction thermodynamics. In addition, the presence of H2O strongly suppresses N2 activation, as traditional catalysts such as Ru and Fe bind H2O more strongly than N2. As a result, direct thermocatalytic NH3 synthesis from N2 and H2O has remained elusive.Direct ammonia synthesis from nitrogen and waterat mild conditions (Image by ZHAO Baibei)In a study published in Journal of the American Chemical Society, a research team led by Prof. DENG Dehui, Prof. HUANG Rui, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved NH3 synthesis directly from N2 and H2O under mild conditions by introducing carbon monoxide (CO) into the reaction system to regulate overall reaction thermodynamics. Using a bifunctional Au/α-MoC1-x catalyst, the team achieves an NH3 production rate of 61 μmolNH3 gcat-1 h-1 at 100 °C, and 1,396 μmolNH3 gcat-1 h-1 at 320 °C, higher than other single-function catalysts.Researchers revealed that the α-MoC1-x phase provides active Mo sites for N2 adsorption and H2O dissociation, while Auδ+ species selectively adsorb CO, promoting the removal of surface oxygen and regenerating active Mo sites. This dual functionality enables a continuous catalytic cycle in which N2 is activated and hydrogenated by in situ-generated OH* species to form NH3."Our study opens new avenues for energy-efficient NH3 production using water as the hydrogen source," said Prof. DENG.
12 18, 2025Researchers Achieve Direct Ammonia Synthesis from Nitrogen and Water under Mild ConditionsA research team led by Prof. DENG Dehui, Prof. HUANG Rui, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved ammonia synthesis directly from nitrogen and water under mild conditions by introducing carbon monoxide into the reaction system to regulate overall reaction thermodynamics.The direct synthesis of ammonia (NH3) from nitrogen (N2) and water (H2O) under mild conditions presents a promising pathway for energy-efficient and sustainable NH3 production. However, thermocatalytic NH3 synthesis from N2 and H2O is limited by unfavorable reaction thermodynamics. In addition, the presence of H2O strongly suppresses N2 activation, as traditional catalysts such as Ru and Fe bind H2O more strongly than N2. As a result, direct thermocatalytic NH3 synthesis from N2 and H2O has remained elusive.Direct ammonia synthesis from nitrogen and waterat mild conditions (Image by ZHAO Baibei)In a study published in Journal of the American Chemical Society, a research team led by Prof. DENG Dehui, Prof. HUANG Rui, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved NH3 synthesis directly from N2 and H2O under mild conditions by introducing carbon monoxide (CO) into the reaction system to regulate overall reaction thermodynamics. Using a bifunctional Au/α-MoC1-x catalyst, the team achieves an NH3 production rate of 61 μmolNH3 gcat-1 h-1 at 100 °C, and 1,396 μmolNH3 gcat-1 h-1 at 320 °C, higher than other single-function catalysts.Researchers revealed that the α-MoC1-x phase provides active Mo sites for N2 adsorption and H2O dissociation, while Auδ+ species selectively adsorb CO, promoting the removal of surface oxygen and regenerating active Mo sites. This dual functionality enables a continuous catalytic cycle in which N2 is activated and hydrogenated by in situ-generated OH* species to form NH3."Our study opens new avenues for energy-efficient NH3 production using water as the hydrogen source," said Prof. DENG. -
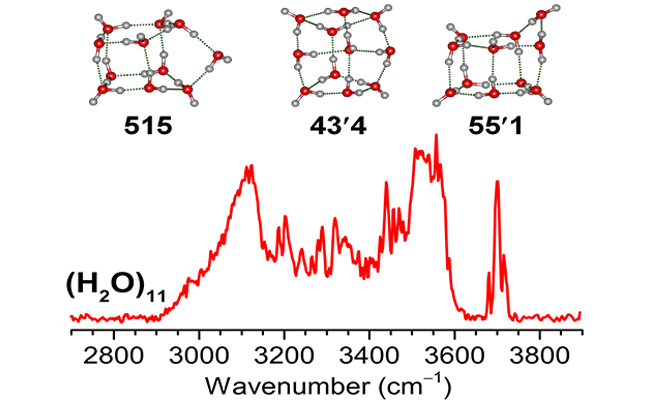 12 16, 2025Researchers Determine the Structural Motifs of the Water Undecamer ClusterA research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University has unveiled experimentally the structure motifs of water undecamer cluster (H2O)11.The constant vibration/rotation and hydrogen-bond (HB) rearrangement of water molecules create various complex yet dynamic HB networks, rendering the structure of liquid water difficult to characterize. Inasmuch as the nature of the intermolecular forces between water molecules in water clusters bears resemblance to that in the bulk, spectroscopic studies of water clusters not only reveals the basic building blocks of the HB network but also provides central benchmarks for developing accurate potential functions and universal models of water.In a study published inNature Communications, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University has unveiled experimentally the structure motifs of water undecamer cluster (H2O)11.Experimental IR spectrum and identified structures of water undecamer cluster (H2O)11 (Image by WANG Tiantong)Profs. JIANG Ling and LI Gang developed a method of infrared spectroscopy of neutral clusters based on a tunable vacuum ultraviolet free electron laser (VUV-FEL). They applied this method to neutral (H2O)11, and recorded the IR spectrum with diverse bands.By combining IR spectrum with high-precision quantum chemical computations from LI Jun's group, three lowest-energy configurations denoted as 515, 43'4, and 55'1 structural motifs were determined. These structures correspond to the "5+1+5", "4+3+4", and "5+5+1" assembling of water cluster pairs, respectively, with the 515 configuration being dominant.Furthermore, the cluster growth mechanisms from water decamer clusters were revealed through thermodynamic analysis. The research offers crucial insights into the evolution of water's HB network and paves the way for size-dependent studies for exploring the stepwise mechanisms of solvation processes such as salt dissolution and acid dissociation.
12 16, 2025Researchers Determine the Structural Motifs of the Water Undecamer ClusterA research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University has unveiled experimentally the structure motifs of water undecamer cluster (H2O)11.The constant vibration/rotation and hydrogen-bond (HB) rearrangement of water molecules create various complex yet dynamic HB networks, rendering the structure of liquid water difficult to characterize. Inasmuch as the nature of the intermolecular forces between water molecules in water clusters bears resemblance to that in the bulk, spectroscopic studies of water clusters not only reveals the basic building blocks of the HB network but also provides central benchmarks for developing accurate potential functions and universal models of water.In a study published inNature Communications, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University has unveiled experimentally the structure motifs of water undecamer cluster (H2O)11.Experimental IR spectrum and identified structures of water undecamer cluster (H2O)11 (Image by WANG Tiantong)Profs. JIANG Ling and LI Gang developed a method of infrared spectroscopy of neutral clusters based on a tunable vacuum ultraviolet free electron laser (VUV-FEL). They applied this method to neutral (H2O)11, and recorded the IR spectrum with diverse bands.By combining IR spectrum with high-precision quantum chemical computations from LI Jun's group, three lowest-energy configurations denoted as 515, 43'4, and 55'1 structural motifs were determined. These structures correspond to the "5+1+5", "4+3+4", and "5+5+1" assembling of water cluster pairs, respectively, with the 515 configuration being dominant.Furthermore, the cluster growth mechanisms from water decamer clusters were revealed through thermodynamic analysis. The research offers crucial insights into the evolution of water's HB network and paves the way for size-dependent studies for exploring the stepwise mechanisms of solvation processes such as salt dissolution and acid dissociation. -
 12 11, 2025Researchers Develop Multiphase 'Soggy Sand' Electrolyte for High-temperature Aqueous Zinc Metal BatteriesA research team led by Prof. CHEN Zhongwei and Prof. WANG Dongdong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed an innovative electrolyte strategy and developed a multiphase aqueous "soggy sand" electrolyte (MASSE), which enhances interfacial stability and electrochemical reversibility of AZMBs at elevated temperatures, enabling stable operation of aqueous batteries in harsh thermal environments.Aqueous zinc metal batteries (AZMBs), known for their high safety, low cost, and environmental friendliness, are considered a promising technology for grid-scale energy storage. However, their practical application has been hindered by hydrogen evolution corrosion at the zinc anode and dissolution of cathode materials, which lead to battery swelling and performance decay. Particularly under extremely high-temperature conditions, where traditional aqueous electrolytes suffer from uncontrollable side reactions above 60 ℃, limiting battery reliability.In a recent study published in Nature Communications, a research team led by Prof. CHEN Zhongwei and Prof. WANG Dongdong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed an innovative electrolyte strategy and developed a multiphase aqueous "soggy sand" electrolyte (MASSE), which enhances interfacial stability and electrochemical reversibility of AZMBs at elevated temperatures, enabling stable operation of aqueous batteries in harsh thermal environments.Researchers constructed MASSE by using dual immobilization of diethylene glycol and aluminum oxide nanoparticles, effectively restricting the activity of free water. The interactions among these multiphase components create a water-deficient solvent structure, endowing MASSE with exceptional thermal stability. Simultaneously, MASSE suppresses water-induced side reactions and promotes uniform zinc ion deposition/stripping even at elevated temperatures.Using MASSE, researchers built a Zn||PANI full cell operating over an ultra-wide temperature range—from room temperature to 140 ℃—with a lifespan of 1,700 cycles at a current density of 8 A g-1. In addition, an aqueous zinc metal pouch cell achieved over 100 stable cycles at 80 ℃, with infrared thermal imaging confirming uniform temperature distribution."The MASSE not only advances high-temperature aqueous batteries, but also provides an innovative electrolyte design strategy for next-generation energy storage systems capable of operating under harsh conditions," said Prof. CHEN.
12 11, 2025Researchers Develop Multiphase 'Soggy Sand' Electrolyte for High-temperature Aqueous Zinc Metal BatteriesA research team led by Prof. CHEN Zhongwei and Prof. WANG Dongdong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed an innovative electrolyte strategy and developed a multiphase aqueous "soggy sand" electrolyte (MASSE), which enhances interfacial stability and electrochemical reversibility of AZMBs at elevated temperatures, enabling stable operation of aqueous batteries in harsh thermal environments.Aqueous zinc metal batteries (AZMBs), known for their high safety, low cost, and environmental friendliness, are considered a promising technology for grid-scale energy storage. However, their practical application has been hindered by hydrogen evolution corrosion at the zinc anode and dissolution of cathode materials, which lead to battery swelling and performance decay. Particularly under extremely high-temperature conditions, where traditional aqueous electrolytes suffer from uncontrollable side reactions above 60 ℃, limiting battery reliability.In a recent study published in Nature Communications, a research team led by Prof. CHEN Zhongwei and Prof. WANG Dongdong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed an innovative electrolyte strategy and developed a multiphase aqueous "soggy sand" electrolyte (MASSE), which enhances interfacial stability and electrochemical reversibility of AZMBs at elevated temperatures, enabling stable operation of aqueous batteries in harsh thermal environments.Researchers constructed MASSE by using dual immobilization of diethylene glycol and aluminum oxide nanoparticles, effectively restricting the activity of free water. The interactions among these multiphase components create a water-deficient solvent structure, endowing MASSE with exceptional thermal stability. Simultaneously, MASSE suppresses water-induced side reactions and promotes uniform zinc ion deposition/stripping even at elevated temperatures.Using MASSE, researchers built a Zn||PANI full cell operating over an ultra-wide temperature range—from room temperature to 140 ℃—with a lifespan of 1,700 cycles at a current density of 8 A g-1. In addition, an aqueous zinc metal pouch cell achieved over 100 stable cycles at 80 ℃, with infrared thermal imaging confirming uniform temperature distribution."The MASSE not only advances high-temperature aqueous batteries, but also provides an innovative electrolyte design strategy for next-generation energy storage systems capable of operating under harsh conditions," said Prof. CHEN. -
 12 08, 2025Copper–Palladium Hydride Interfaces Enable Highly Efficient Electrochemical Ammonia SynthesisA research team led by Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a copper-palladium (CuPd) bimetallic catalyst with abundant Cu–PdHx interfaces. These formed hydride interfaces enhance intrinsic activity, enabling efficient, stable, and scalable nitrate-to-ammonia electrolysis in membrane electrode assembly (MEA) electrolyzers.Ammonia (NH3) is essential for global agriculture and plays an important role in next-generation carbon-free energy systems. As a supplementary or alternative to the traditional Haber-Bosch process, renewable ammonia synthesis has become a major technological pursuit.Among the emerging routes, the electrochemical nitrate reduction reaction (NO3−RR) to ammonia offers a promising route for sustainable ammonia production as well as effective nitrogen recovery. However, slow reaction kinetics and the competing hydrogen evolution reaction (HER) continue to hinder efficient ammonia synthesis.In a recent study published in Nature Synthesis, a research team led by Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a copper-palladium (CuPd) bimetallic catalyst with abundant Cu–PdHx interfaces. These formed hydride interfaces enhance intrinsic activity, enabling efficient, stable, and scalable nitrate-to-ammonia electrolysis in membrane electrode assembly (MEA) electrolyzers.Copper–palladium hydride interfaces achieve efficient and stable nitrate electrolysis in a membrane electrode assembly electrolyzer (Image by FU Yunfan and WANG Shuo)Under NO3−RR operating conditions, the CuPd bimetallic catalyst exhibits high intrinsic catalytic activity, achieving an NH3 production rate of 19.9 mmol h−1 cm−2 with a current density of 5 A cm–2 at a full-cell voltage of 2.56 V in an MEA electrolyzer. The stability test further demonstrated good durability, maintaining a Faradaic efficiency of about 86.8% at 2 A cm−2 for over 1,000 hours.Researchers further revealed that the enhanced performance is attributed to the superior intrinsic activity of the Cu–PdHx interfaces. The hydrogen redistribution induced by overflow at the Cu–PdHx interface modifies the local electronic structure of the active sites. This optimizes NO3− adsorption, promotes NH3 desorption, and provides a more energetically favorable reaction pathway for ammonia synthesis.Moreover, a scale-up demonstration using an electrolyzer stack with five 100 cm2 MEAs achieved an NH3 production rate of 8.7 mol h–1 at 500 A. It also continuously delivered 1.6 mol h–1 of ammonia at 100 A for 100 hours, underscoring its industrial potential.This study provides new insight into the structure-activity relationship of CuPd bimetallic sites and suggests an effective strategy for enhancing intrinsic catalytic activity through the in situ construction of beneficial interfaces, enabling efficient conversion of nitrate pollutants into value-added ammonia.
12 08, 2025Copper–Palladium Hydride Interfaces Enable Highly Efficient Electrochemical Ammonia SynthesisA research team led by Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a copper-palladium (CuPd) bimetallic catalyst with abundant Cu–PdHx interfaces. These formed hydride interfaces enhance intrinsic activity, enabling efficient, stable, and scalable nitrate-to-ammonia electrolysis in membrane electrode assembly (MEA) electrolyzers.Ammonia (NH3) is essential for global agriculture and plays an important role in next-generation carbon-free energy systems. As a supplementary or alternative to the traditional Haber-Bosch process, renewable ammonia synthesis has become a major technological pursuit.Among the emerging routes, the electrochemical nitrate reduction reaction (NO3−RR) to ammonia offers a promising route for sustainable ammonia production as well as effective nitrogen recovery. However, slow reaction kinetics and the competing hydrogen evolution reaction (HER) continue to hinder efficient ammonia synthesis.In a recent study published in Nature Synthesis, a research team led by Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a copper-palladium (CuPd) bimetallic catalyst with abundant Cu–PdHx interfaces. These formed hydride interfaces enhance intrinsic activity, enabling efficient, stable, and scalable nitrate-to-ammonia electrolysis in membrane electrode assembly (MEA) electrolyzers.Copper–palladium hydride interfaces achieve efficient and stable nitrate electrolysis in a membrane electrode assembly electrolyzer (Image by FU Yunfan and WANG Shuo)Under NO3−RR operating conditions, the CuPd bimetallic catalyst exhibits high intrinsic catalytic activity, achieving an NH3 production rate of 19.9 mmol h−1 cm−2 with a current density of 5 A cm–2 at a full-cell voltage of 2.56 V in an MEA electrolyzer. The stability test further demonstrated good durability, maintaining a Faradaic efficiency of about 86.8% at 2 A cm−2 for over 1,000 hours.Researchers further revealed that the enhanced performance is attributed to the superior intrinsic activity of the Cu–PdHx interfaces. The hydrogen redistribution induced by overflow at the Cu–PdHx interface modifies the local electronic structure of the active sites. This optimizes NO3− adsorption, promotes NH3 desorption, and provides a more energetically favorable reaction pathway for ammonia synthesis.Moreover, a scale-up demonstration using an electrolyzer stack with five 100 cm2 MEAs achieved an NH3 production rate of 8.7 mol h–1 at 500 A. It also continuously delivered 1.6 mol h–1 of ammonia at 100 A for 100 hours, underscoring its industrial potential.This study provides new insight into the structure-activity relationship of CuPd bimetallic sites and suggests an effective strategy for enhancing intrinsic catalytic activity through the in situ construction of beneficial interfaces, enabling efficient conversion of nitrate pollutants into value-added ammonia. -
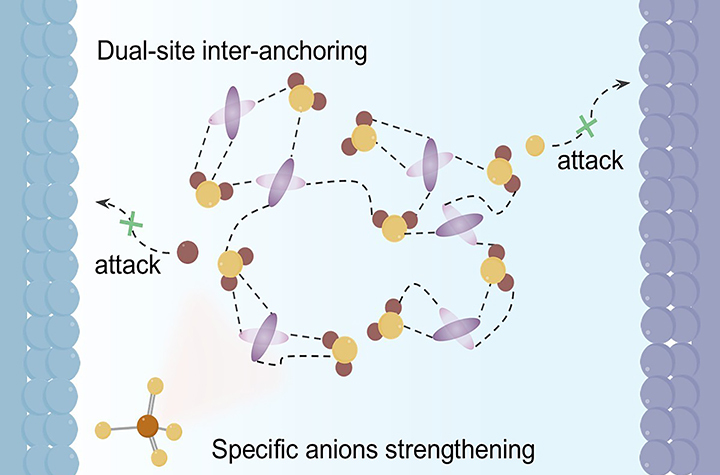 12 05, 2025Researchers Construct Robust Inter-Anchored Hydrogen-Bond Network for Long-Life Aqueous Zinc-ion BatteriesA research group team led by Prof. YANG Weishen and Prof. ZHU Kaiyue from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Science (CAS) constructed a robust hydrogen-bond network within in the electrolyte, which minimizinge the reactivity of both hydrogen (H) and oxygen (O) atoms in water.Aqueous zinc-ion batteries (ZIBs), known for their intrinsic safety, low cost, and high ionic conductivity, have emerged as promising candidates for large-scale energy storage systems.Among the commonly used cathode materials, vanadium-based cathodes stand out due to their high capacity, simple energy-storage mechanism, and good compatibility with high mass loading. However, the strong reactivity of water molecules in the electrolyte leads to severe vanadium dissolution at the cathode, hydrogen evolution, and zinc (Zn) corrosion at the anode, which hinders the long-lasting cycling performance of ZIBs, especially at low current density.Reinforced dual-site H-bond anchoring suppresses water reactivity, effectively reducing V dissolution and hydrogen evolution in aqueous ZIBs (Image by OU Zuqiao)In a recent study published in Angewandte Chemie International Edition, a research team led by Prof. YANG Weishen and Prof. ZHU Kaiyue from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Science (CAS) constructed a robust hydrogen-bond network within the electrolyte, minimizing the reactivity of both hydrogen (H) and oxygen (O) atoms in water. This design suppresses deterioration on the bilateral electrode.To build this robust H-bond network, the researchers employed ethylene glycol as a cosolvent and sulfate ion (SO42-) as structure-making anions. Owing to its abundant H-bond acceptors and donors, ethylene glycol provides a dual-site H-bond anchoring effect on active water molecules, thus effectively impeding the incursion of both H and O atoms towards both electrodes. Notably, the ion-specific, structure-making capability of SO42- further strengthens this dual-site anchoring, reducing vanadium dissolution at the cathode.Moreover, ethylene glycol-induced modulation of Zn2+ solvation structures accelerates Zn2+ desolvation kinetics at the cathode and enhances the (de)intercalation reversibility of both Zn2+ and H+. Benefitting from these synergistic effects, ZIBs using the ethylene glycol-containing electrolyte achieve long-lasting cycling stability, retaining 87% capacity retention after 500 cycles at 0.5 A g-1.Furthermore, the optimized electrolyte enabled a 90 cm2 pouch cell to deliver a high capacity of 2 Ah at 4 A, while retaining 80% capacity after 70 cycles, underscoring its strong practical potential.
12 05, 2025Researchers Construct Robust Inter-Anchored Hydrogen-Bond Network for Long-Life Aqueous Zinc-ion BatteriesA research group team led by Prof. YANG Weishen and Prof. ZHU Kaiyue from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Science (CAS) constructed a robust hydrogen-bond network within in the electrolyte, which minimizinge the reactivity of both hydrogen (H) and oxygen (O) atoms in water.Aqueous zinc-ion batteries (ZIBs), known for their intrinsic safety, low cost, and high ionic conductivity, have emerged as promising candidates for large-scale energy storage systems.Among the commonly used cathode materials, vanadium-based cathodes stand out due to their high capacity, simple energy-storage mechanism, and good compatibility with high mass loading. However, the strong reactivity of water molecules in the electrolyte leads to severe vanadium dissolution at the cathode, hydrogen evolution, and zinc (Zn) corrosion at the anode, which hinders the long-lasting cycling performance of ZIBs, especially at low current density.Reinforced dual-site H-bond anchoring suppresses water reactivity, effectively reducing V dissolution and hydrogen evolution in aqueous ZIBs (Image by OU Zuqiao)In a recent study published in Angewandte Chemie International Edition, a research team led by Prof. YANG Weishen and Prof. ZHU Kaiyue from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Science (CAS) constructed a robust hydrogen-bond network within the electrolyte, minimizing the reactivity of both hydrogen (H) and oxygen (O) atoms in water. This design suppresses deterioration on the bilateral electrode.To build this robust H-bond network, the researchers employed ethylene glycol as a cosolvent and sulfate ion (SO42-) as structure-making anions. Owing to its abundant H-bond acceptors and donors, ethylene glycol provides a dual-site H-bond anchoring effect on active water molecules, thus effectively impeding the incursion of both H and O atoms towards both electrodes. Notably, the ion-specific, structure-making capability of SO42- further strengthens this dual-site anchoring, reducing vanadium dissolution at the cathode.Moreover, ethylene glycol-induced modulation of Zn2+ solvation structures accelerates Zn2+ desolvation kinetics at the cathode and enhances the (de)intercalation reversibility of both Zn2+ and H+. Benefitting from these synergistic effects, ZIBs using the ethylene glycol-containing electrolyte achieve long-lasting cycling stability, retaining 87% capacity retention after 500 cycles at 0.5 A g-1.Furthermore, the optimized electrolyte enabled a 90 cm2 pouch cell to deliver a high capacity of 2 Ah at 4 A, while retaining 80% capacity after 70 cycles, underscoring its strong practical potential. -
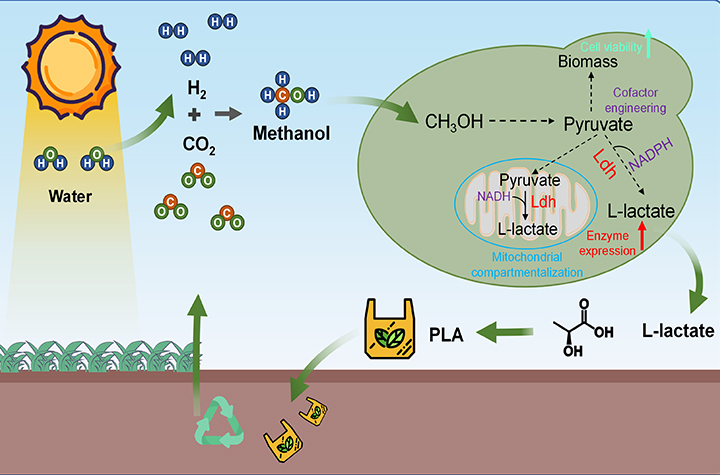 12 01, 2025Yeast Cell Factory Developed to Convert Methanol into L-lactateA research team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. FEI Qiang from Xi'an Jiaotong University, has developed a yeast cell factory to produce L-lactate from methanol as the sole carbon source.Methanol is an ideal feedstock for bio-manufacturing. Converting it into lactate, a monomer for biodegradable plastic, offers a promising strategy for addressing the challenge of white pollution. However, it remains difficult to engineer microbes to produce lactate from methanol due to methanol toxicity and strong competition between product synthesis and cell growth.Multi-dimensional engineering of a methylotrophic yeast (Image by Yu Wei)In a study published in Nature Communications, a research team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. FEI Qiang from Xi'an Jiaotong University, developed a yeast cell factory to produce L-lactate from methanol as the sole carbon source, and evaluated the commercial potential and environmental impacts of this bioprocess.Researchers extensively rewired the metabolism of the yeast Ogataea polymorpha to enable overproduction of L-lactate from methanol and discovered a distinct cofactor distribution pattern in methanol metabolism. Based on this cofactor regulation mechanism, they developed cofactor engineering and mitochondrial compartmentalization strategies, substantially enhancing L-lactate biosynthesis.Through techno-economic analysis and life cycle assessment, researchers showed that the minimum selling price of L-lactate produced from methanol was 2.29 dollar/kg with the annual capacity of 18,500 tons. They also showed that the synthesis of 1 ton of L-lactate resulted in a carbon deposition of 7.29 tons, realizing the carbon-negative production. These demonstrate the economic and environmental value of producing CO2-derived L-lactate by coupling chemical and biological catalysis."Our work not only paves the way for engineering methanol metabolism to produce lactate, but also shows a broader vision for a circular economy by establishing a direct link between biodegradable plastics and carbon neutrality," said Prof. ZHOU.
12 01, 2025Yeast Cell Factory Developed to Convert Methanol into L-lactateA research team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. FEI Qiang from Xi'an Jiaotong University, has developed a yeast cell factory to produce L-lactate from methanol as the sole carbon source.Methanol is an ideal feedstock for bio-manufacturing. Converting it into lactate, a monomer for biodegradable plastic, offers a promising strategy for addressing the challenge of white pollution. However, it remains difficult to engineer microbes to produce lactate from methanol due to methanol toxicity and strong competition between product synthesis and cell growth.Multi-dimensional engineering of a methylotrophic yeast (Image by Yu Wei)In a study published in Nature Communications, a research team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. FEI Qiang from Xi'an Jiaotong University, developed a yeast cell factory to produce L-lactate from methanol as the sole carbon source, and evaluated the commercial potential and environmental impacts of this bioprocess.Researchers extensively rewired the metabolism of the yeast Ogataea polymorpha to enable overproduction of L-lactate from methanol and discovered a distinct cofactor distribution pattern in methanol metabolism. Based on this cofactor regulation mechanism, they developed cofactor engineering and mitochondrial compartmentalization strategies, substantially enhancing L-lactate biosynthesis.Through techno-economic analysis and life cycle assessment, researchers showed that the minimum selling price of L-lactate produced from methanol was 2.29 dollar/kg with the annual capacity of 18,500 tons. They also showed that the synthesis of 1 ton of L-lactate resulted in a carbon deposition of 7.29 tons, realizing the carbon-negative production. These demonstrate the economic and environmental value of producing CO2-derived L-lactate by coupling chemical and biological catalysis."Our work not only paves the way for engineering methanol metabolism to produce lactate, but also shows a broader vision for a circular economy by establishing a direct link between biodegradable plastics and carbon neutrality," said Prof. ZHOU.