Aqueous zinc-ion batteries (AZIBs) are regarded as promising next-generation solution for large-scale energy storage, offering advantages such as high safety, low cost, and environmental friendliness. However, under practical high-rate and long-cycling conditions, Zn anodes suffer from interfacial challenges–including hydrogen evolution, self-corrosion, and dendrite growth–that hinder performance and life of the batteries.
In a recent study published in Angewandte Chemie International Edition, Prof. CHEN Zhongwei's team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences proposed a novel π-electron delocalization-based strategy for additive molecule screening. The team established a clear structure–function relationship between additive molecular design and interfacial behavior, providing a new avenue for optimizing AZIB performance.
By leveraging the self-assembly behavior of trace functional organic molecules at the electrode interface, the researchers constructed a flexible hydrophilic–hydrophobic interfacial layer (HHIL) on the Zn anode surface. This HHIL enhances interfacial stability and electrochemical reversibility, opening a new pathway toward high-performance AZIBs.
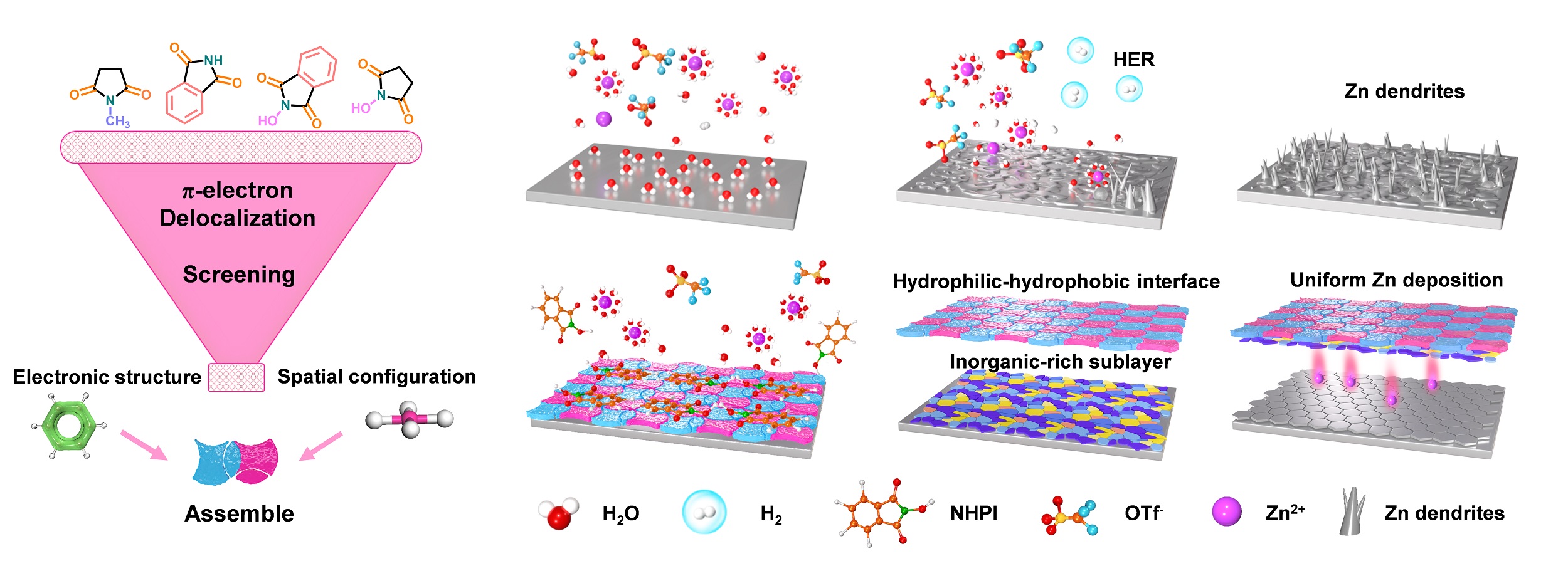
Molecular structure screening of additives and the schematic diagram of severe HER, chemical corrosion, non-uniform Zn2+ deposition, and dendrite growth on the Zn anode in BE (Image by SANG Yudong)
The researchers identified N-hydroxyphthalimide (NHPI) as a functional additive, featuring a rigid quasi-planar structure, strong π-electron delocalization, and a high positive electrostatic potential. Through π–π stacking and ion–dipole interactions, NHPI spontaneously self-assemble on the Zn surface to form a robust HHIL. During cycling, this HHIL further promotes the formation of an inorganic sublayer rich in ZnF2 and ZnS, resulting in a dual-layer structure that combines flexible organic and rigid inorganic interfaces.
This cooperative interface effectively regulatess Zn2+ deposition, suppresses parasitic reactions and dendrite growth, and enhances interfacial stability.
As a result, Zn//Zn symmetric cells achieved stable cycling over 900 hours at a high current density of 20 mA cm-2 with a capacity of 10 mAh cm-2. Moreover, full Zn//NaV3O8·1.5H2O (NVO) cells delivered more than 25,000 cycles at 10 A g-1 with 85% capacity retention, outperforming baseline systems. The strategy also demonstrated high rate performance and practical applicability in soft-pack pouch cells.
"Our study establishes a full-link mechanism from molecular design to interfacial construction to performance enhancement," said Prof. CHEN. "It offers both theoretical insights and experimental evidence for additive molecule design and interface regulation in AZIBs, accelerating their development for long-life, high-energy-density energy storage applications," added Prof. CHEN.