For decades, aluminosilicate and silicophosphoaluminate zeolites were considered as inert materials for molecular hydrogen activation as well as hydrogenation reaction.
However, recent studies have shown that metal-free zeolites and zeotypes can also catalyze olefins hydrogenation and the ring-opening of aromatic, which leads to key impact on the products selectivity and stability. Despite these observations, the nature of the active site responsible for H2 activation and the mechanism of olefin hydrogenation within zeolites and zeotypes remain unclear.
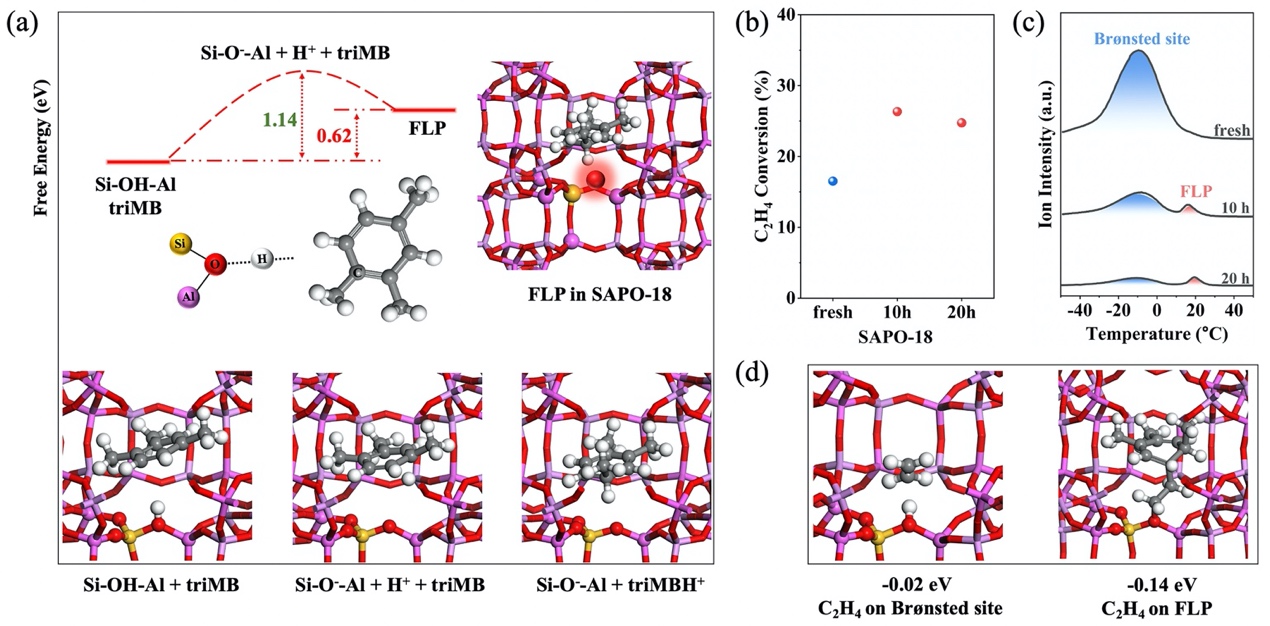
Formation of FLP sites over SAPO-18 and their catalytic hydrogenation of ethylene (Image by LI Mengyuan)
In a study published in the Journal of American Chemical Society, a research group led by Prof. JIAO Feng and Prof. PAN Xiulian from the Dalian Institute of Chemical Physics(DICP) of the Chinese Academy of Sciences (CAS) discovered that Brønsted acid sites in zeolites can form frustrated Lewis pairs (FLPs) with confined carbon species, thereby regulating the hydrogenation of intermediate olefins.
The researchers found that Brønsted acid sites with relatively weaker acidity can transfer a proton (H+) to nearby carbon species, generating a Si-O⁻-Al site as the Lewis base and a carbocation as the Lewis acid. Their close spatial proximity creates electrostatic attraction and steric hindrance, leading to the formation of FLPs. Moreover, the researchers confirmed the formation of these FLP sites, demonstrated their ability to activate H-H bond, and proposed general design principles for FLP generation within zeolites.
"Our study not only offers new insights into the hydrogenation mechanism of olefins catalyzed by metal-free zeolite materials, but also provides theoretical foundations for the development of high-performance zeolite-based catalysts and heterogeneous FLP catalytic materials," said Prof. JIAO.