Research News
-
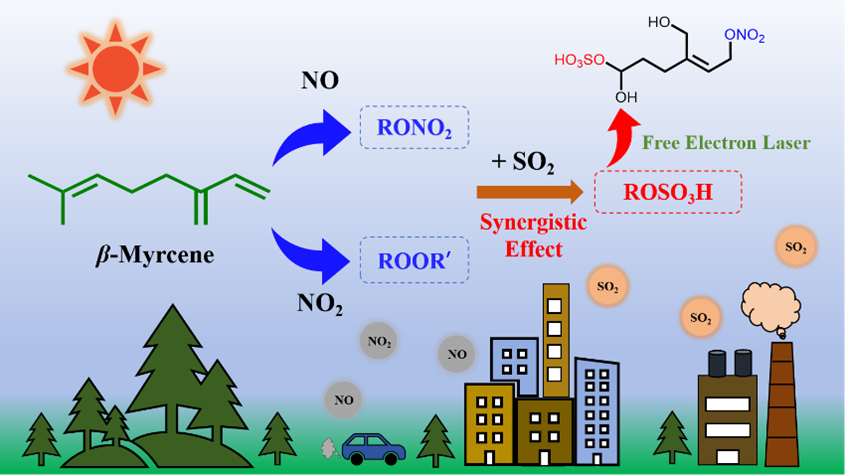 02 03, 2026Researchers Reveal Distinct Roles of NO2 versus NO and Synergisms with SO2 on β-Myrcene PhotooxidationA research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed distinct roles of NOx (NO2 and NO), as well as their synergistic interactions with SO2, in SOA formation during β-myrcene photooxidation.Atmospheric particulate pollutants pose threats to both human health and ecosystems. According to Atmospheric China 2025: Best Practices in Air Pollution Prevention and Control in China, although China's annual average PM2.5 concentration now meets the National Ambient Air Quality Standard (35 μg/m3), it remains higher than the World Health Organization's guideline (5 μg/m3).The formation of aerosols from molecular precursors is a key process driving particulate pollution, making precise characterization of their chemical composition and nucleation mechanisms essential for elucidating formation pathways and informing effective control measures. As priority pollutants in air quality management, nitrogen oxides (NOx = NO + NO2) and sulfur dioxide (SO2) play critical yet incompletely understood roles in secondary organic aerosol (SOA) formation from volatile organic compounds.In a study published in Environmental Science & Technology, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed distinct roles of NOx (NO2 and NO), as well as their synergistic interactions with SO2, in SOA formation during β-myrcene photooxidation.Schematic illustration of the synergistic effects of NOx and SO2 on β-myrcene photooxidation (Image by ZHAO Ya)Using a DICP-CAS smog chamber with advanced online analytical instruments, the researchers investigated the SOA formation from β-myrcene photooxidation. The results revealed a significant difference between the effects of NO2 and NO. NO2 enhanced SOA yield and increased the oxygen-to-carbon (O:C) ratio through oxidant amplification. In contrast, NO suppressed particle nucleation and reduced product oxidation states, while promoting particle growth by facilitating the partitioning of organic nitrates into the particulate phase. SO2 exhibited strong synergistic effects with both NO2 and NO, facilitating particle formation and growth.Furthermore, the researchers developed a novel aerosol mass spectrometer based on a tunable vacuum ultraviolet free electron laser (VUV-FEL). The newly-built instrument enabled the detection of previously unobserved compounds, including organic peroxides, organic nitrate, and organosulfates. Combined with quantum chemical computations, the researchers proposed detailed oxidation pathways for the formation of these compounds."The identification and analysis of these novel compounds help to correct the biases in SOA formation predictions and provide more precise data for understanding aerosol formation mechanisms in regions influenced by both anthropogenic and biogenic sources," said Prof. JIANG.
02 03, 2026Researchers Reveal Distinct Roles of NO2 versus NO and Synergisms with SO2 on β-Myrcene PhotooxidationA research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed distinct roles of NOx (NO2 and NO), as well as their synergistic interactions with SO2, in SOA formation during β-myrcene photooxidation.Atmospheric particulate pollutants pose threats to both human health and ecosystems. According to Atmospheric China 2025: Best Practices in Air Pollution Prevention and Control in China, although China's annual average PM2.5 concentration now meets the National Ambient Air Quality Standard (35 μg/m3), it remains higher than the World Health Organization's guideline (5 μg/m3).The formation of aerosols from molecular precursors is a key process driving particulate pollution, making precise characterization of their chemical composition and nucleation mechanisms essential for elucidating formation pathways and informing effective control measures. As priority pollutants in air quality management, nitrogen oxides (NOx = NO + NO2) and sulfur dioxide (SO2) play critical yet incompletely understood roles in secondary organic aerosol (SOA) formation from volatile organic compounds.In a study published in Environmental Science & Technology, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed distinct roles of NOx (NO2 and NO), as well as their synergistic interactions with SO2, in SOA formation during β-myrcene photooxidation.Schematic illustration of the synergistic effects of NOx and SO2 on β-myrcene photooxidation (Image by ZHAO Ya)Using a DICP-CAS smog chamber with advanced online analytical instruments, the researchers investigated the SOA formation from β-myrcene photooxidation. The results revealed a significant difference between the effects of NO2 and NO. NO2 enhanced SOA yield and increased the oxygen-to-carbon (O:C) ratio through oxidant amplification. In contrast, NO suppressed particle nucleation and reduced product oxidation states, while promoting particle growth by facilitating the partitioning of organic nitrates into the particulate phase. SO2 exhibited strong synergistic effects with both NO2 and NO, facilitating particle formation and growth.Furthermore, the researchers developed a novel aerosol mass spectrometer based on a tunable vacuum ultraviolet free electron laser (VUV-FEL). The newly-built instrument enabled the detection of previously unobserved compounds, including organic peroxides, organic nitrate, and organosulfates. Combined with quantum chemical computations, the researchers proposed detailed oxidation pathways for the formation of these compounds."The identification and analysis of these novel compounds help to correct the biases in SOA formation predictions and provide more precise data for understanding aerosol formation mechanisms in regions influenced by both anthropogenic and biogenic sources," said Prof. JIANG. -
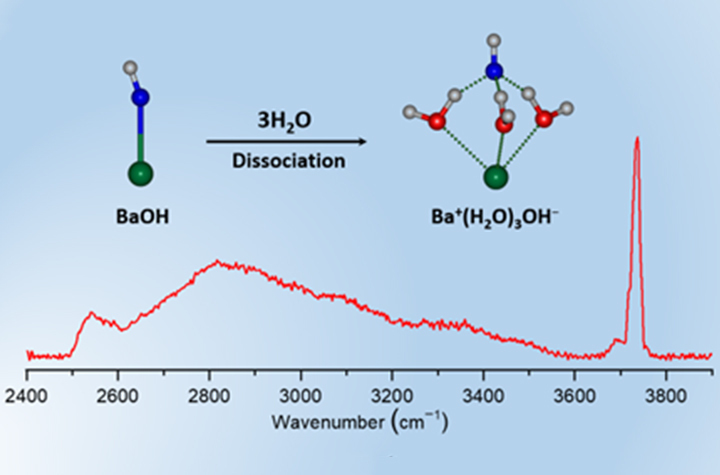 01 15, 2026Researchers Unveil Microscopic Mechanism of Alkali Species Dissolution in Water ClustersA research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has experimentally revealed that three water molecules can separate Ba and OH in neutral BaOH(H2O)n (n = 1−5) clusters, uncovering the microscopic mechanism of alkali dissolution exemplified by hydrated BaOH clusters.The dissolution of alkali species in water is a fundamental process in acid–base chemistry and plays a crucial role in a wide range of applications, from energy storage to pharmaceuticals.Understanding how water molecules initiate the dissociation of alkali at the molecular level has been a long-standing challenge due to the difficulty in probing hydrogen bonding, proton transfer, and electrostatic interactions in complex solvent environments. Neutral hydrated alkali clusters serve as ideal models for investigating these early solvation processes, yet their spectroscopic study has been hindered by the lack of charge, making them difficult to detect and mass-select.In a study published in the Journal of the American Chemical Society, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has experimentally revealed that three water molecules can separate Ba and OH in neutral BaOH(H2O)n (n = 1−5) clusters, uncovering the microscopic mechanism of alkali dissolution exemplified by hydrated BaOH clusters.Experimental IR-VUV spectra and identified structures of hydrated BaOH clusters (Image by YAN Wenhui)Researchers developed a neutral cluster infrared spectroscopy station based on infrared excitation and vacuum ultraviolet threshold photoionization (IR-VUV). This setup enables high-sensitivity IR spectral detection, structural characterization, and reactivity studies of mass-selected neutral clusters. Utilizing this platform along with tabletop extreme ultraviolet sources, the team measured the IR spectra of neutral BaOH(H2O)n (n = 1−5) clusters.By comparing experimental spectra with high-level quantum chemical harmonic calculations and anharmonic molecular dynamics simulations, the researchers found that when n = 1 and 2, water molecules interact directly with BaOH via O–H⋯O hydrogen bonds without dissociation of Ba and OH. When n ≥ 3, Ba and OH dissociate to form a solvent-shared ion pair structure. Electronic structure analysis revealed that as the number of water molecules increases, charge transfer reduces electrostatic attraction, and the formation of a hydrogen-bond network promotes the separation of Ba and OH.This work provides critical insights into the early solvation in closed-shell systems and establishes a model for understanding electrostatic and inductive interactions between ionic species and water molecules. The findings pave the way for further size-resolved studies of solvation mechanisms in chemical and biological processes.
01 15, 2026Researchers Unveil Microscopic Mechanism of Alkali Species Dissolution in Water ClustersA research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has experimentally revealed that three water molecules can separate Ba and OH in neutral BaOH(H2O)n (n = 1−5) clusters, uncovering the microscopic mechanism of alkali dissolution exemplified by hydrated BaOH clusters.The dissolution of alkali species in water is a fundamental process in acid–base chemistry and plays a crucial role in a wide range of applications, from energy storage to pharmaceuticals.Understanding how water molecules initiate the dissociation of alkali at the molecular level has been a long-standing challenge due to the difficulty in probing hydrogen bonding, proton transfer, and electrostatic interactions in complex solvent environments. Neutral hydrated alkali clusters serve as ideal models for investigating these early solvation processes, yet their spectroscopic study has been hindered by the lack of charge, making them difficult to detect and mass-select.In a study published in the Journal of the American Chemical Society, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has experimentally revealed that three water molecules can separate Ba and OH in neutral BaOH(H2O)n (n = 1−5) clusters, uncovering the microscopic mechanism of alkali dissolution exemplified by hydrated BaOH clusters.Experimental IR-VUV spectra and identified structures of hydrated BaOH clusters (Image by YAN Wenhui)Researchers developed a neutral cluster infrared spectroscopy station based on infrared excitation and vacuum ultraviolet threshold photoionization (IR-VUV). This setup enables high-sensitivity IR spectral detection, structural characterization, and reactivity studies of mass-selected neutral clusters. Utilizing this platform along with tabletop extreme ultraviolet sources, the team measured the IR spectra of neutral BaOH(H2O)n (n = 1−5) clusters.By comparing experimental spectra with high-level quantum chemical harmonic calculations and anharmonic molecular dynamics simulations, the researchers found that when n = 1 and 2, water molecules interact directly with BaOH via O–H⋯O hydrogen bonds without dissociation of Ba and OH. When n ≥ 3, Ba and OH dissociate to form a solvent-shared ion pair structure. Electronic structure analysis revealed that as the number of water molecules increases, charge transfer reduces electrostatic attraction, and the formation of a hydrogen-bond network promotes the separation of Ba and OH.This work provides critical insights into the early solvation in closed-shell systems and establishes a model for understanding electrostatic and inductive interactions between ionic species and water molecules. The findings pave the way for further size-resolved studies of solvation mechanisms in chemical and biological processes. -
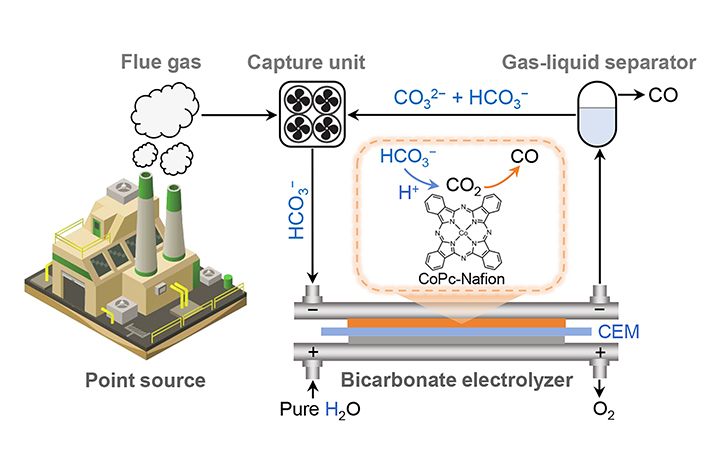 01 13, 2026Researchers Achieve Efficient Bicarbonate-Mediated Integrated Capture and Electrolysis of Carbon DioxideA research team led by Profs. BAO Xinhe, GAO Dunfeng, and ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) in collaboration with Prof. WANG Guoxiong from Fudan University achieved efficient bicarbonate-mediated integrated CO2 capture and electrolysis to CO through an ionomer-driven reaction microenvironment control strategy.The capture and conversion of carbon dioxide (CO2) from industrial flue gas is a promising carbon capture, utilization, and storage (CCUS) process. Traditional routes typically follow a "capture-release-compression-electrolysis" tandem pathway, which is complex and energy-intensive. As an emerging reactive carbon capture technology, the bicarbonate-mediated integrated CO2 capture-electrolysis route couples upstream CO2 capture with subsequent electrocatalytic conversion, reducing the energy consumption associated with obtaining high-purity CO2 feedstock.The electrolysis of bicarbonate capture liquids is a crucial step in the bicarbonate-mediated integrated CO2 capture-electrolysis route. However, this step suffers from insufficient current density (low reaction rate) and high cell voltage (low energy efficiency) .In a study published in Angewandte Chemie International Edition, a research team led by Profs. BAO Xinhe, GAO Dunfeng, and ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) in collaboration with Prof. WANG Guoxiong from Fudan University achieved efficient bicarbonate-mediated integrated CO2 capture and electrolysis to CO through an ionomer-driven reaction microenvironment control strategy.Ionomer-driven reaction microenvironment control in bicarbonate-mediated integrated CO2 capture and electrolysis (Image by RONG Youwen)Researchers improved the bicarbonate electrolysis performance by manipulating reaction microenvironments by introducing ionomers into cobalt phthalocyanine (CoPc) electrodes. In a cation exchange membrane-based zero-gap electrolyzer, the CoPc electrode modified with a Nafion ionomer exhibits a high CO Faradaic efficiency of 93% at an applied current density of 300 mA cm−2 and a CO partial current density of 410 mA cm−2 at a low cell voltage of 3.09 V.Electrode structure characterization and finite element simulation results indicated that the proton conductivity of the Nafion ionomer increases the local concentration of in situ generated CO2 (i-CO2) in the proximity of the CoPc catalyst, resulting in improved CO formation.Furthermore, researchers demonstrated a closed-loop CO2 capture and electrolysis cycle at the device level using the Nafion-incorporated CoPc electrode and a simulated flue gas."Our study showcases the promise of the reaction microenvironment control strategy for improving bicarbonate electrolysis performance and advancing reactive carbon capture technology," said Prof. GAO.
01 13, 2026Researchers Achieve Efficient Bicarbonate-Mediated Integrated Capture and Electrolysis of Carbon DioxideA research team led by Profs. BAO Xinhe, GAO Dunfeng, and ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) in collaboration with Prof. WANG Guoxiong from Fudan University achieved efficient bicarbonate-mediated integrated CO2 capture and electrolysis to CO through an ionomer-driven reaction microenvironment control strategy.The capture and conversion of carbon dioxide (CO2) from industrial flue gas is a promising carbon capture, utilization, and storage (CCUS) process. Traditional routes typically follow a "capture-release-compression-electrolysis" tandem pathway, which is complex and energy-intensive. As an emerging reactive carbon capture technology, the bicarbonate-mediated integrated CO2 capture-electrolysis route couples upstream CO2 capture with subsequent electrocatalytic conversion, reducing the energy consumption associated with obtaining high-purity CO2 feedstock.The electrolysis of bicarbonate capture liquids is a crucial step in the bicarbonate-mediated integrated CO2 capture-electrolysis route. However, this step suffers from insufficient current density (low reaction rate) and high cell voltage (low energy efficiency) .In a study published in Angewandte Chemie International Edition, a research team led by Profs. BAO Xinhe, GAO Dunfeng, and ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) in collaboration with Prof. WANG Guoxiong from Fudan University achieved efficient bicarbonate-mediated integrated CO2 capture and electrolysis to CO through an ionomer-driven reaction microenvironment control strategy.Ionomer-driven reaction microenvironment control in bicarbonate-mediated integrated CO2 capture and electrolysis (Image by RONG Youwen)Researchers improved the bicarbonate electrolysis performance by manipulating reaction microenvironments by introducing ionomers into cobalt phthalocyanine (CoPc) electrodes. In a cation exchange membrane-based zero-gap electrolyzer, the CoPc electrode modified with a Nafion ionomer exhibits a high CO Faradaic efficiency of 93% at an applied current density of 300 mA cm−2 and a CO partial current density of 410 mA cm−2 at a low cell voltage of 3.09 V.Electrode structure characterization and finite element simulation results indicated that the proton conductivity of the Nafion ionomer increases the local concentration of in situ generated CO2 (i-CO2) in the proximity of the CoPc catalyst, resulting in improved CO formation.Furthermore, researchers demonstrated a closed-loop CO2 capture and electrolysis cycle at the device level using the Nafion-incorporated CoPc electrode and a simulated flue gas."Our study showcases the promise of the reaction microenvironment control strategy for improving bicarbonate electrolysis performance and advancing reactive carbon capture technology," said Prof. GAO. -
 01 12, 2026Researchers Achieve Chain-Length Control of Fatty Acid Biosynthesis in YeastA collaborative team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and Prof. Martin Grininger from Goethe University Frankfurt developed a modular and programmable fatty acid synthesis platform that enables high specificity production of medium-chain fatty acids in yeast.Medium- and short-chain fatty acids (C8–C14) are widely used in industries including food, pharmaceuticals, lubricants, and surfactants, and they are currently mainly extracted from coconut and palm oils. Developing sustainable microbial alternatives, especially for producing fatty acids with high purity and precise chain-length control, is a major goal of synthetic biology and metabolic engineering.In a study published in Nature Chemical Biology, Prof. ZHOU Yongjin's team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and Prof. Martin Grininger's team from Goethe University Frankfurt developed a modular and programmable fatty acid synthesis platform which enables high specificity production of medium-chain fatty acids in yeast.Engineering metazoan fatty acid synthase for chain-length control in yeast (Image by ZHAI Xiaoxin)Prof. Grininger's team engineered a metazoan fatty acid synthase (mFAS) through targeted mutations, which can modulate the activity of its ketosynthase (KS) and thioesterase (TesA) domains to precisely control the fatty acid chain length.Prof. ZHOU's team constructed an efficient yeast cell factory using the industrial yeast Ogataea polymorpha. The optimized mFAS/TesA system was integrated and co-expressed alongside engineered fatty acid metabolism. These modifications blocked the complete degradation of long-chain fatty acids and redirected metabolic flux toward medium-chain products.The engineered strain XMCFA69, an engineered Ogataea polymorpha strain developed in this study, achieved a medium-chain fatty acid titer of 708.6 mg/L, with lauric acid (C12) accounting for 48% of the total products, which was comparable to its abundance in coconut and palm kernel oils."This study develops a programmable, chain-length-controllable platform through synergistic enzyme and metabolic engineering. It demonstrates the potential of synthetic biology for sustainable chemical manufacturing, and offers a viable alternative to plant-based fatty acid extraction," said Prof. ZHOU.
01 12, 2026Researchers Achieve Chain-Length Control of Fatty Acid Biosynthesis in YeastA collaborative team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and Prof. Martin Grininger from Goethe University Frankfurt developed a modular and programmable fatty acid synthesis platform that enables high specificity production of medium-chain fatty acids in yeast.Medium- and short-chain fatty acids (C8–C14) are widely used in industries including food, pharmaceuticals, lubricants, and surfactants, and they are currently mainly extracted from coconut and palm oils. Developing sustainable microbial alternatives, especially for producing fatty acids with high purity and precise chain-length control, is a major goal of synthetic biology and metabolic engineering.In a study published in Nature Chemical Biology, Prof. ZHOU Yongjin's team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and Prof. Martin Grininger's team from Goethe University Frankfurt developed a modular and programmable fatty acid synthesis platform which enables high specificity production of medium-chain fatty acids in yeast.Engineering metazoan fatty acid synthase for chain-length control in yeast (Image by ZHAI Xiaoxin)Prof. Grininger's team engineered a metazoan fatty acid synthase (mFAS) through targeted mutations, which can modulate the activity of its ketosynthase (KS) and thioesterase (TesA) domains to precisely control the fatty acid chain length.Prof. ZHOU's team constructed an efficient yeast cell factory using the industrial yeast Ogataea polymorpha. The optimized mFAS/TesA system was integrated and co-expressed alongside engineered fatty acid metabolism. These modifications blocked the complete degradation of long-chain fatty acids and redirected metabolic flux toward medium-chain products.The engineered strain XMCFA69, an engineered Ogataea polymorpha strain developed in this study, achieved a medium-chain fatty acid titer of 708.6 mg/L, with lauric acid (C12) accounting for 48% of the total products, which was comparable to its abundance in coconut and palm kernel oils."This study develops a programmable, chain-length-controllable platform through synergistic enzyme and metabolic engineering. It demonstrates the potential of synthetic biology for sustainable chemical manufacturing, and offers a viable alternative to plant-based fatty acid extraction," said Prof. ZHOU. -
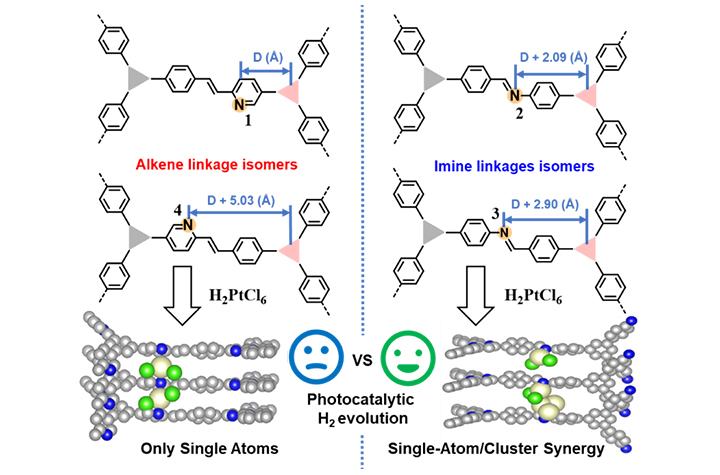 12 25, 2025Researchers Enhance Photocatalytic Hydrogen Evolution Performance of Covalent Organic Frameworks by ConstitutionalIsomer StrategyProf. ZHOU Xukai and his colleagues from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) enhanced the photocatalytic hydrogen evolution performance of covalent organic frameworks (COFs) by introducing a conformational isomer strategy to precisely tune the nitrogen atom positions.Photocatalytic hydrogen evolution is a key technology for clean energy conversion, in which platinum (Pt) is widely used as an effective cocatalyst. The anchoring and dispersion of Pt play a decisive role in catalytic performance. However, achieving precise control over metal-support interactions at the atomic level remains challenging due to the chemical heterogeneity of catalyst surfaces.In a recent study published in Angewandte Chemie International Edition, Prof. ZHOU Xukai and his colleagues from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) enhanced the photocatalytic hydrogen evolution performance of covalent organic frameworks (COFs) by introducing a conformational isomer strategy to precisely tune the nitrogen atom positions.Constitutional isomerism in alkene/imine-linked COFs enables Ångström-scale positional control of N-anchoring sites (yellow) along hexagonal channel arms, dictating distinct Pt deposition configurations (single atoms/clusters) via in situ photodeposition of H2PtCl6 (Image by LI Hanxi and ZHOU Xukai)COFs, with their programmable topology and well-defined pore environments, provide an ideal platform for studying metal-support interactions at the atomic level. In this work, researchers designed four COFs by combining trisubstituted aldehydes with trisubstituted aromatic amines or aromatic methyl compounds, yielding two olefin-linked COFs (COF-A1/A2) and two imine-linked COFs (COF-I1/I2). Although these materials share the same hexagonal pore topology, the positions of nitrogen anchoring sites were precisely tuned at the angstrom-level. After in situ photodeposition of Pt, the spatial arrangement of nitrogen atoms was found to play a decisive role in determining the dispersion and coordination environment of Pt species.Multiple characterizations revealed that the imine-linked COF-I2 can simultaneously stabilize both Pt2+ single atoms and metallic Pt clusters, forming dual active sites. In contrast, the olefin-linked COF-A2 primarily anchors Pt single atoms. This structural difference directly leads to a remarkable disparity in photocatalytic performance: COF-I2-Pt exhibits a high hydrogen evolution rate of 26.72 mmol h-1 g-1, which is 6.1 times higher than that of its olefin-linked counterpart, COF-A2-Pt (4.40 mmol h-1 g-1). Under monochromatic light irradiation at 420 nm, COF-I2-Pt achieves an apparent quantum efficiency of 12.1%.Further mechanistic investigations revealed that the superior performance of COF-I2-Pt stems from the synergistic effect between Pt clusters and single atoms. The Pt clusters act as hole-relay centers, while single atoms serve as electron-trapping sites. The charge redistribution between them effectively promotes the separation of photogenerated electron-hole pairs and optimizes the kinetics of proton adsorption and reduction. Femtosecond transient absorption spectroscopy further confirms a prolonged lifetime of the key charge-separated state in COF-I2-Pt, which is the primary reason for its efficient hydrogen evolution."Our study provides a new route for the rational design of atomically precise photocatalysts," said Prof. ZHOU. "And the concept of 'nitrogen-shift engineering' can be extended to the design of other porous framework materials, offering important guidance for developing efficient energy conversion materials."
12 25, 2025Researchers Enhance Photocatalytic Hydrogen Evolution Performance of Covalent Organic Frameworks by ConstitutionalIsomer StrategyProf. ZHOU Xukai and his colleagues from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) enhanced the photocatalytic hydrogen evolution performance of covalent organic frameworks (COFs) by introducing a conformational isomer strategy to precisely tune the nitrogen atom positions.Photocatalytic hydrogen evolution is a key technology for clean energy conversion, in which platinum (Pt) is widely used as an effective cocatalyst. The anchoring and dispersion of Pt play a decisive role in catalytic performance. However, achieving precise control over metal-support interactions at the atomic level remains challenging due to the chemical heterogeneity of catalyst surfaces.In a recent study published in Angewandte Chemie International Edition, Prof. ZHOU Xukai and his colleagues from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) enhanced the photocatalytic hydrogen evolution performance of covalent organic frameworks (COFs) by introducing a conformational isomer strategy to precisely tune the nitrogen atom positions.Constitutional isomerism in alkene/imine-linked COFs enables Ångström-scale positional control of N-anchoring sites (yellow) along hexagonal channel arms, dictating distinct Pt deposition configurations (single atoms/clusters) via in situ photodeposition of H2PtCl6 (Image by LI Hanxi and ZHOU Xukai)COFs, with their programmable topology and well-defined pore environments, provide an ideal platform for studying metal-support interactions at the atomic level. In this work, researchers designed four COFs by combining trisubstituted aldehydes with trisubstituted aromatic amines or aromatic methyl compounds, yielding two olefin-linked COFs (COF-A1/A2) and two imine-linked COFs (COF-I1/I2). Although these materials share the same hexagonal pore topology, the positions of nitrogen anchoring sites were precisely tuned at the angstrom-level. After in situ photodeposition of Pt, the spatial arrangement of nitrogen atoms was found to play a decisive role in determining the dispersion and coordination environment of Pt species.Multiple characterizations revealed that the imine-linked COF-I2 can simultaneously stabilize both Pt2+ single atoms and metallic Pt clusters, forming dual active sites. In contrast, the olefin-linked COF-A2 primarily anchors Pt single atoms. This structural difference directly leads to a remarkable disparity in photocatalytic performance: COF-I2-Pt exhibits a high hydrogen evolution rate of 26.72 mmol h-1 g-1, which is 6.1 times higher than that of its olefin-linked counterpart, COF-A2-Pt (4.40 mmol h-1 g-1). Under monochromatic light irradiation at 420 nm, COF-I2-Pt achieves an apparent quantum efficiency of 12.1%.Further mechanistic investigations revealed that the superior performance of COF-I2-Pt stems from the synergistic effect between Pt clusters and single atoms. The Pt clusters act as hole-relay centers, while single atoms serve as electron-trapping sites. The charge redistribution between them effectively promotes the separation of photogenerated electron-hole pairs and optimizes the kinetics of proton adsorption and reduction. Femtosecond transient absorption spectroscopy further confirms a prolonged lifetime of the key charge-separated state in COF-I2-Pt, which is the primary reason for its efficient hydrogen evolution."Our study provides a new route for the rational design of atomically precise photocatalysts," said Prof. ZHOU. "And the concept of 'nitrogen-shift engineering' can be extended to the design of other porous framework materials, offering important guidance for developing efficient energy conversion materials." -
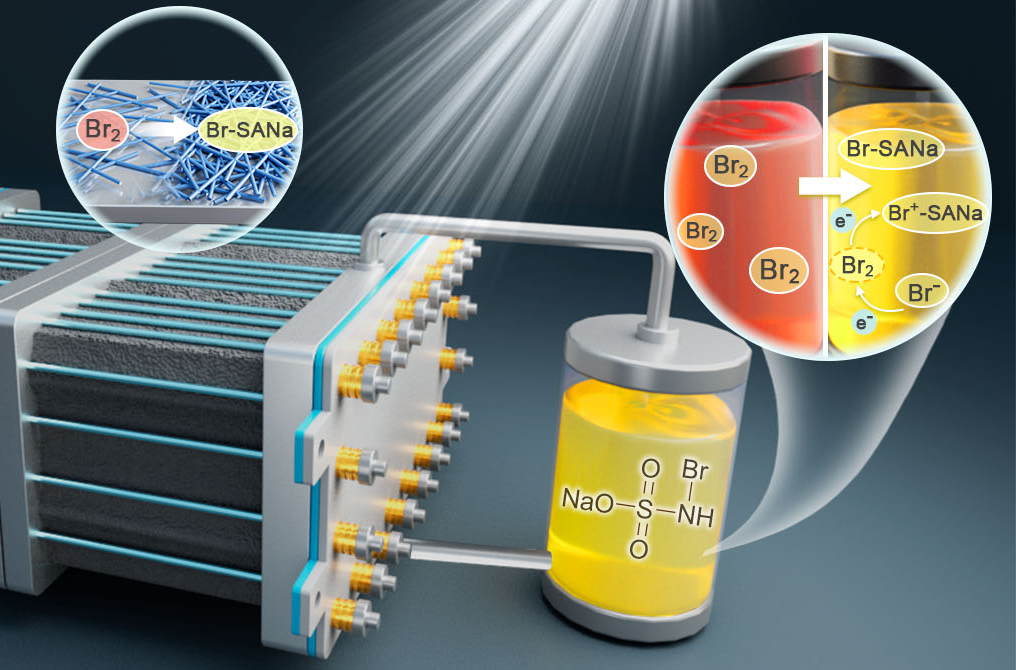 12 20, 2025Researchers Develop New System for High-energy-density, Long-life, Multi-electron Transfer Bromine-based Flow BatteriesA research team led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a novel bromine-based two-electron transfer reaction system and applied it to a zinc-bromine flow battery.Bromine-based flow batteries operate through the redox reaction between bromide ions and elemental bromine, offering advantages such as abundant resources, high redox potential, and good solubility. However, the substantial bromine generated during the charging process can corrode battery components, shorten cycle life, and increase system costs. Although traditional bromine complexing agents can alleviate corrosion to some extent, they often induce phase separation, compromising electrolyte homogeneity and adding complexity to the system.In a recent study published in Nature Energy, a research team led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a novel bromine-based two-electron transfer reaction system and applied it to a zinc-bromine flow battery. This study demonstrates both the proof of concept and the system-scale up of a long-life zinc-bromine flow battery.Researchers proposed an innovative bromine two-electron transfer reaction by introducing amine compounds as bromine scavengers into the electrolyte. They discovered that the bromine (Br2) generated during the electrochemical reaction could be converted into brominated amine compounds, reducing the concentration of Br2 in the solution to an ultra-low level of approximately 7 mM. Unlike the traditional single-electron transfer reaction from bromide ions to Br2, this new reaction enables a two-electron transfer from bromide ions to brominated amine compounds, increasing the battery's energy density.Simultaneously, the ultra-low Br2 concentration substantially reduces electrolyte corrosivity, extending battery life.The principle and battery verification of the novel bromine-based two-electron transfer reaction system (Image by XIE Congxin Xie and XU Yue)Furthermore, researchers applied this novel reaction to zinc-bromine flow batteries. Due to the extremely low Br2 concentration in the electrolyte, the long-term stable operation was achieved by assembling a single battery using a conventional non-fluorinated ion exchange membrane (SPEEK), thereby reducing battery costs. In a 5 kW system scale-up test, the battery operated stably for over 700 cycles under a current density of 40 mA cm-2, achieving an energy efficiency above 78%. With the drastically reduced Br2 concentration, no corrosion was observed in key materials—including current collectors, electrodes, and membranes—either before or after cycling."Our study provides a novel approach to the design of long-life bromine-based flow batteries and lays the foundation for the further application and promotion of zinc-bromine flow batteries," said Prof. LI.
12 20, 2025Researchers Develop New System for High-energy-density, Long-life, Multi-electron Transfer Bromine-based Flow BatteriesA research team led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a novel bromine-based two-electron transfer reaction system and applied it to a zinc-bromine flow battery.Bromine-based flow batteries operate through the redox reaction between bromide ions and elemental bromine, offering advantages such as abundant resources, high redox potential, and good solubility. However, the substantial bromine generated during the charging process can corrode battery components, shorten cycle life, and increase system costs. Although traditional bromine complexing agents can alleviate corrosion to some extent, they often induce phase separation, compromising electrolyte homogeneity and adding complexity to the system.In a recent study published in Nature Energy, a research team led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a novel bromine-based two-electron transfer reaction system and applied it to a zinc-bromine flow battery. This study demonstrates both the proof of concept and the system-scale up of a long-life zinc-bromine flow battery.Researchers proposed an innovative bromine two-electron transfer reaction by introducing amine compounds as bromine scavengers into the electrolyte. They discovered that the bromine (Br2) generated during the electrochemical reaction could be converted into brominated amine compounds, reducing the concentration of Br2 in the solution to an ultra-low level of approximately 7 mM. Unlike the traditional single-electron transfer reaction from bromide ions to Br2, this new reaction enables a two-electron transfer from bromide ions to brominated amine compounds, increasing the battery's energy density.Simultaneously, the ultra-low Br2 concentration substantially reduces electrolyte corrosivity, extending battery life.The principle and battery verification of the novel bromine-based two-electron transfer reaction system (Image by XIE Congxin Xie and XU Yue)Furthermore, researchers applied this novel reaction to zinc-bromine flow batteries. Due to the extremely low Br2 concentration in the electrolyte, the long-term stable operation was achieved by assembling a single battery using a conventional non-fluorinated ion exchange membrane (SPEEK), thereby reducing battery costs. In a 5 kW system scale-up test, the battery operated stably for over 700 cycles under a current density of 40 mA cm-2, achieving an energy efficiency above 78%. With the drastically reduced Br2 concentration, no corrosion was observed in key materials—including current collectors, electrodes, and membranes—either before or after cycling."Our study provides a novel approach to the design of long-life bromine-based flow batteries and lays the foundation for the further application and promotion of zinc-bromine flow batteries," said Prof. LI.