Ammonia (NH3) is essential for global agriculture and plays an important role in next-generation carbon-free energy systems. As a supplementary or alternative to the traditional Haber-Bosch process, renewable ammonia synthesis has become a major technological pursuit.
Among the emerging routes, the electrochemical nitrate reduction reaction (NO3−RR) to ammonia offers a promising route for sustainable ammonia production as well as effective nitrogen recovery. However, slow reaction kinetics and the competing hydrogen evolution reaction (HER) continue to hinder efficient ammonia synthesis.
In a recent study published in Nature Synthesis, a research team led by Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a copper-palladium (CuPd) bimetallic catalyst with abundant Cu–PdHx interfaces. These formed hydride interfaces enhance intrinsic activity, enabling efficient, stable, and scalable nitrate-to-ammonia electrolysis in membrane electrode assembly (MEA) electrolyzers.
Copper–palladium hydride interfaces achieve efficient and stable nitrate electrolysis in a membrane electrode assembly electrolyzer (Image by FU Yunfan and WANG Shuo)
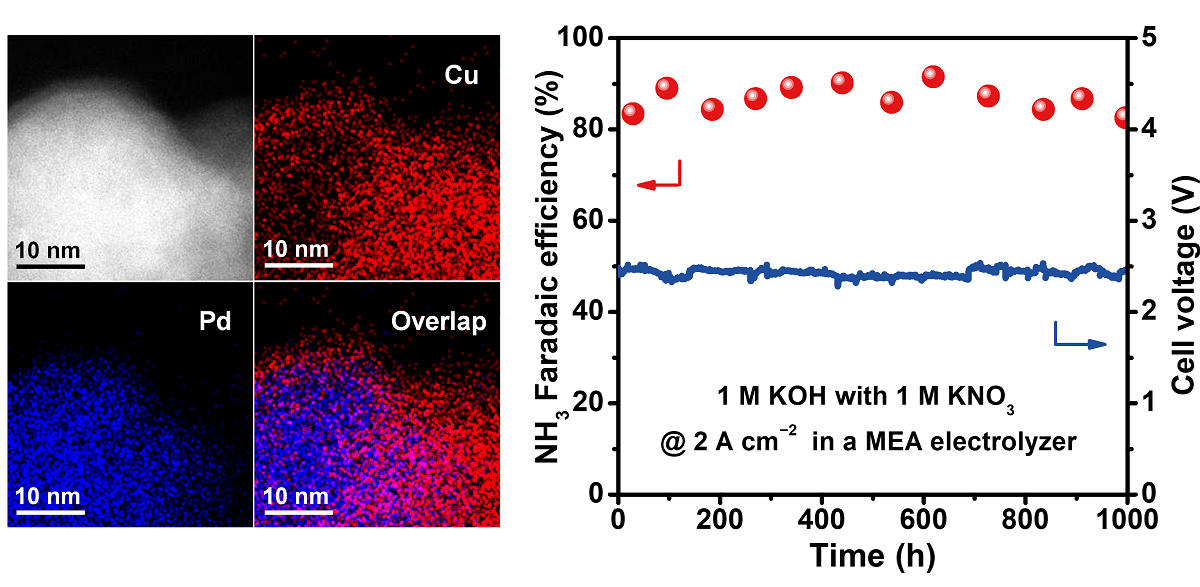
Under NO3−RR operating conditions, the CuPd bimetallic catalyst exhibits high intrinsic catalytic activity, achieving an NH3 production rate of 19.9 mmol h−1 cm−2 with a current density of 5 A cm–2 at a full-cell voltage of 2.56 V in an MEA electrolyzer. The stability test further demonstrated good durability, maintaining a Faradaic efficiency of about 86.8% at 2 A cm−2 for over 1,000 hours.
Researchers further revealed that the enhanced performance is attributed to the superior intrinsic activity of the Cu–PdHx interfaces. The hydrogen redistribution induced by overflow at the Cu–PdHx interface modifies the local electronic structure of the active sites. This optimizes NO3− adsorption, promotes NH3 desorption, and provides a more energetically favorable reaction pathway for ammonia synthesis.
Moreover, a scale-up demonstration using an electrolyzer stack with five 100 cm2 MEAs achieved an NH3 production rate of 8.7 mol h–1 at 500 A. It also continuously delivered 1.6 mol h–1 of ammonia at 100 A for 100 hours, underscoring its industrial potential.
This study provides new insight into the structure-activity relationship of CuPd bimetallic sites and suggests an effective strategy for enhancing intrinsic catalytic activity through the in situ construction of beneficial interfaces, enabling efficient conversion of nitrate pollutants into value-added ammonia.