Research News
-
 11 28, 2025Researchers Develop Real-world Data-driven Framework for Accurate Electric Vehicle Range Prediction and Intelligent ManagementA research team led by Prof. CHEN Zhongwei and Associate Prof. MAO Zhiyu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Associate Prof. ZHANG Zhaosheng from the Beijing Institute of Technology, developed a comprehensive real-world data–driven framework for EVs range prediction and intelligent management."Range anxiety" remains one of the major issues of electric vehicles (EVs). Most of the existing range prediction technologies rely on simulated conditions or limited datasets, making it difficult to accurately capture variations caused by regional climate, road conditions, and vehicle types.In a recent study published in Applied Energy, a research team led by Prof. CHEN Zhongwei and Associate Prof. MAO Zhiyu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Associate Prof. ZHANG Zhaosheng from the Beijing Institute of Technology, developed a comprehensive real-world data–driven framework for EVs range prediction and intelligent management.The researchers developed an integrated framework for online range estimation and optimization analysis which incorporates the influence including driving behavior, ambient temperature, and battery State of Health (SOH). A Random Forest algorithm was used to predict energy consumption per unit distance and the remaining range, which improves prediction accuracy and enhances model interpretability.The remaining driving range estimation and analysis framework for electric vehicles (Image by ZHOU Litao)Using three years of real-world operational data with a combined driving distance of more than 300,000 kilometers, the researchers validated the framework. They found that the average relative error between the predicted remaining driving range (RDR) and the actual drivable distance is below 5.5%, outperforming traditional methods.Further analysis showed that the average current which reflects the power intensity of the trip and average speed are the key determinants of energy consumption. Reasonable adjustments in driving behavior could increase the driving range by more than 30% for passenger cars and more than 10% for buses."Our study not only answers the question of how far the vehicle can go, but also provides a quantitative basis for how to go farther," said Prof. CHEN. The study achieves a high-accuracy RDR estimation under diverse, real-world operating conditions, and provides an engineering-ready solution for intelligent EV fleet management.
11 28, 2025Researchers Develop Real-world Data-driven Framework for Accurate Electric Vehicle Range Prediction and Intelligent ManagementA research team led by Prof. CHEN Zhongwei and Associate Prof. MAO Zhiyu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Associate Prof. ZHANG Zhaosheng from the Beijing Institute of Technology, developed a comprehensive real-world data–driven framework for EVs range prediction and intelligent management."Range anxiety" remains one of the major issues of electric vehicles (EVs). Most of the existing range prediction technologies rely on simulated conditions or limited datasets, making it difficult to accurately capture variations caused by regional climate, road conditions, and vehicle types.In a recent study published in Applied Energy, a research team led by Prof. CHEN Zhongwei and Associate Prof. MAO Zhiyu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Associate Prof. ZHANG Zhaosheng from the Beijing Institute of Technology, developed a comprehensive real-world data–driven framework for EVs range prediction and intelligent management.The researchers developed an integrated framework for online range estimation and optimization analysis which incorporates the influence including driving behavior, ambient temperature, and battery State of Health (SOH). A Random Forest algorithm was used to predict energy consumption per unit distance and the remaining range, which improves prediction accuracy and enhances model interpretability.The remaining driving range estimation and analysis framework for electric vehicles (Image by ZHOU Litao)Using three years of real-world operational data with a combined driving distance of more than 300,000 kilometers, the researchers validated the framework. They found that the average relative error between the predicted remaining driving range (RDR) and the actual drivable distance is below 5.5%, outperforming traditional methods.Further analysis showed that the average current which reflects the power intensity of the trip and average speed are the key determinants of energy consumption. Reasonable adjustments in driving behavior could increase the driving range by more than 30% for passenger cars and more than 10% for buses."Our study not only answers the question of how far the vehicle can go, but also provides a quantitative basis for how to go farther," said Prof. CHEN. The study achieves a high-accuracy RDR estimation under diverse, real-world operating conditions, and provides an engineering-ready solution for intelligent EV fleet management. -
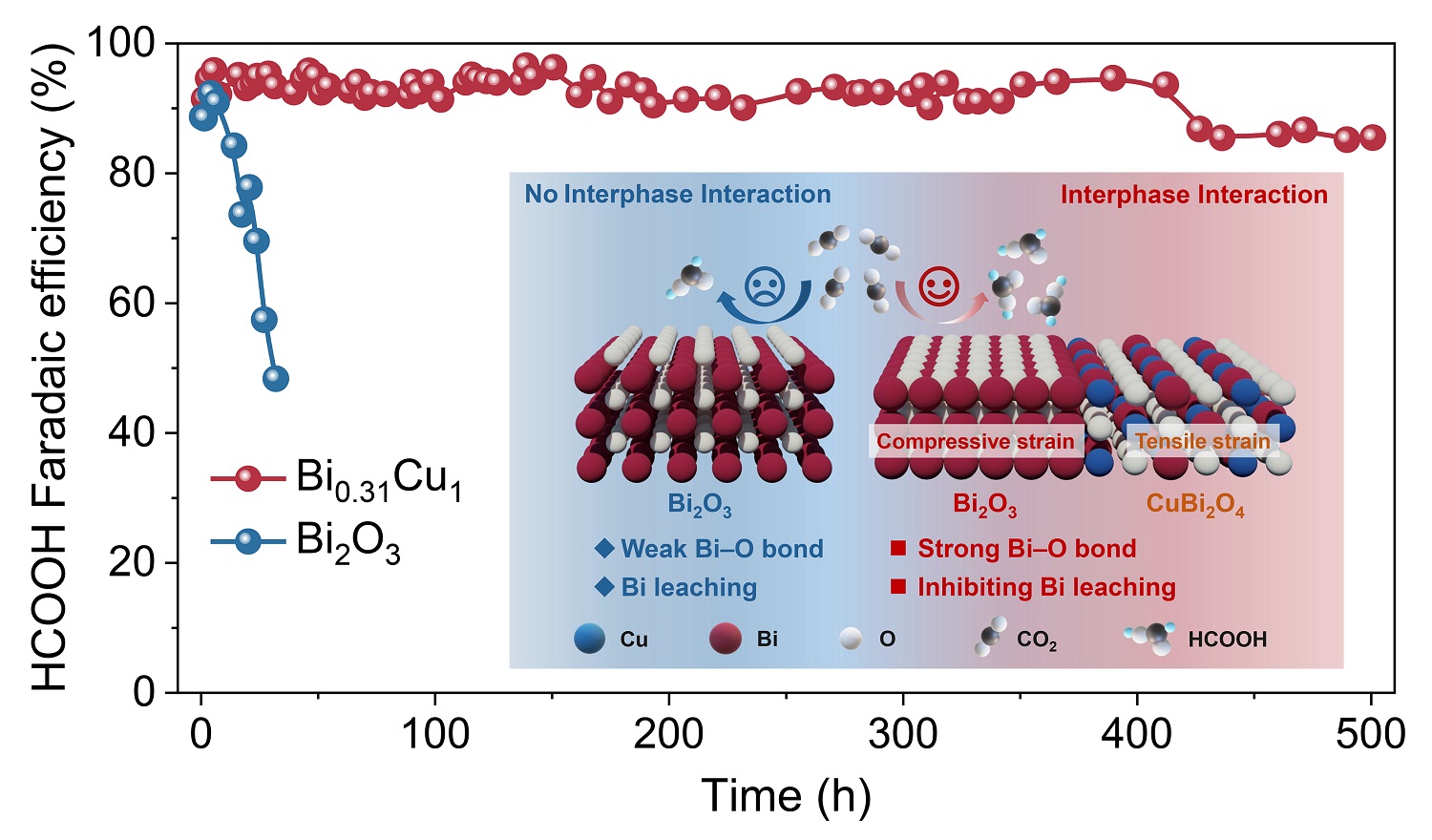 11 18, 2025Researchers Achieve Durable Acidic Carbon Dioxide Electroreduction to Formic Acid through Metal-phase Protection StrategyA research team led by Prof. GAO Dunfeng, in collaboration with Prof. ZHOU Xukai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved durable acidic CO2 electroreduction to formic acid through a metal-phase protection strategy.The electrocatalytic reduction of carbon dioxide (CO2) into valuable chemicals and fuels provides a sustainable route to address global climate and energy challenges. However, CO2 electroreduction typically operates under alkaline or neutral conditions, where the carbonation side reaction causes carbon loss. Moreover, the main product— formate—requires additional post-treatment steps, such as acidification, to obtain formic acid.Acidic CO2 electroreduction can effectively mitigate carbonation issues and directly produce formic acid, but harsh acidic environments often lead to metal leaching and rapid catalyst degradation, limiting both activity and long-term durability.In a study published in Angewandte Chemie International Edition, a research team led by Prof. GAO Dunfeng, in collaboration with Prof. ZHOU Xukai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved durable acidic CO2 electroreduction to formic acid through a metal-phase protection strategy.Metal-phase protection suppresses Bi leaching for durable acidic CO2 electroreduction to formic acid (Image by TAN Zijian)Researchers proposed a metal-phase protection strategy that enabled the in situ formation of a Bi-Cu bimetallic oxide catalyst (Bi0.31Cu1). This catalyst exhibits high activity and durability for acidic CO2 electroreduction to formic acid (HCOOH). The Bi0.31Cu1 catalyst delivers a Faradaic efficiency for HCOOH above 90% in a wide current density range from 200 to 650 mA cm−2. In a 0.5 M KCl electrolyte at pH 2, the Bi0.31Cu1 catalyst can continuously produce HCOOH with approximately 90% Faradaic efficiency at 200 mA cm−2 for 500 hours.Furthermore, researchers revealed that the interphase interaction between the Bi2O3 phase and the CuBi2O4 phase within the Bi0.31Cu1 catalyst induces compressive strain and lattice contraction in the Bi2O3 phase. This strengthens the Bi−O bond, effectively suppressing Bi leaching during catalyst reconstruction and thus delivering both high activity and long-term durability. The general applicability of this metal-phase protection strategy was further demonstrated using a Bi-Mg bimetallic catalyst."Our study showcases the promise of the metal-phase protection strategy for developing highly efficient catalysts with long-term durability for acidic CO2 electrolysis," said Prof. GAO.
11 18, 2025Researchers Achieve Durable Acidic Carbon Dioxide Electroreduction to Formic Acid through Metal-phase Protection StrategyA research team led by Prof. GAO Dunfeng, in collaboration with Prof. ZHOU Xukai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved durable acidic CO2 electroreduction to formic acid through a metal-phase protection strategy.The electrocatalytic reduction of carbon dioxide (CO2) into valuable chemicals and fuels provides a sustainable route to address global climate and energy challenges. However, CO2 electroreduction typically operates under alkaline or neutral conditions, where the carbonation side reaction causes carbon loss. Moreover, the main product— formate—requires additional post-treatment steps, such as acidification, to obtain formic acid.Acidic CO2 electroreduction can effectively mitigate carbonation issues and directly produce formic acid, but harsh acidic environments often lead to metal leaching and rapid catalyst degradation, limiting both activity and long-term durability.In a study published in Angewandte Chemie International Edition, a research team led by Prof. GAO Dunfeng, in collaboration with Prof. ZHOU Xukai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved durable acidic CO2 electroreduction to formic acid through a metal-phase protection strategy.Metal-phase protection suppresses Bi leaching for durable acidic CO2 electroreduction to formic acid (Image by TAN Zijian)Researchers proposed a metal-phase protection strategy that enabled the in situ formation of a Bi-Cu bimetallic oxide catalyst (Bi0.31Cu1). This catalyst exhibits high activity and durability for acidic CO2 electroreduction to formic acid (HCOOH). The Bi0.31Cu1 catalyst delivers a Faradaic efficiency for HCOOH above 90% in a wide current density range from 200 to 650 mA cm−2. In a 0.5 M KCl electrolyte at pH 2, the Bi0.31Cu1 catalyst can continuously produce HCOOH with approximately 90% Faradaic efficiency at 200 mA cm−2 for 500 hours.Furthermore, researchers revealed that the interphase interaction between the Bi2O3 phase and the CuBi2O4 phase within the Bi0.31Cu1 catalyst induces compressive strain and lattice contraction in the Bi2O3 phase. This strengthens the Bi−O bond, effectively suppressing Bi leaching during catalyst reconstruction and thus delivering both high activity and long-term durability. The general applicability of this metal-phase protection strategy was further demonstrated using a Bi-Mg bimetallic catalyst."Our study showcases the promise of the metal-phase protection strategy for developing highly efficient catalysts with long-term durability for acidic CO2 electrolysis," said Prof. GAO. -
 11 17, 2025Lifespan Engineering Strengthens Yeast Cell Factories for Enhanced BiosynthesisProf. ZHOU Yongjin's team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has demonstrate that combining lifespan engineering strategies with metabolic pathway optimization in Saccharomyces cerevisiae enables highly efficient sclareol biosynthesis.Sclareol is a valuable diterpene alcohol extracted from Salvia sclarea. It is widely used in pharmaceuticals and agrochemicals, and serves as a key precursor for synthesizing the high-value fragrance compound ambergris.While metabolic engineering has enabled the construction of efficient microbial cell factories, cellular aging and the accumulation of toxic metabolites during prolonged fed-batch fermentation induce metabolic stress, ultimately reducing cell productivity. Therefore, extending cellular lifespan represents an effective strategy to enhance biosynthetic capacity.A research team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has achieved an advance in improving microbial production through lifespan engineering. Their findings, published in Proceedings of the National Academy of Sciences of the United States of America, demonstrate that combining lifespan engineering strategies with metabolic pathway optimization in Saccharomyces cerevisiae enables highly efficient sclareol biosynthesis.Building on the high-producing sclareol strain, the researchers systematically engineered cellular lifespan through four dimensions: nutrient sensing, mitophagy, protein stability, and genomic stability. Specifically, simultaneously weakening nutrient sensing and enhancing mitophagy, together with metabolic pathway optimization, resulted in a sclareol production of 25.9 g/L. Omics analysis showed that these strategies enhanced central metabolism and cellular robustness by extending chronological lifespan and regulating metabolic gene expression, thereby improving product synthesis during the later stages of cell growth.In addition, this lifespan engineering strategy also improved the biosynthesis of other products, such as sesquiterpenes and phenolic acids, indicating its broad applicability and providing a generalizable approach for developing high-performance microbial cell factories."Our work not only establishes a clear connection between chronological lifespan and biosynthesis capacity for improving sclareol production," said Prof. ZHOU, "but also offers a feasible longevity engineering strategy that can be extended to diverse microbial cell factories for sustainable and economical biomanufacturing."Lifespan engineering strategy (Image by WU Zulin and GAO Jiaoqi)
11 17, 2025Lifespan Engineering Strengthens Yeast Cell Factories for Enhanced BiosynthesisProf. ZHOU Yongjin's team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has demonstrate that combining lifespan engineering strategies with metabolic pathway optimization in Saccharomyces cerevisiae enables highly efficient sclareol biosynthesis.Sclareol is a valuable diterpene alcohol extracted from Salvia sclarea. It is widely used in pharmaceuticals and agrochemicals, and serves as a key precursor for synthesizing the high-value fragrance compound ambergris.While metabolic engineering has enabled the construction of efficient microbial cell factories, cellular aging and the accumulation of toxic metabolites during prolonged fed-batch fermentation induce metabolic stress, ultimately reducing cell productivity. Therefore, extending cellular lifespan represents an effective strategy to enhance biosynthetic capacity.A research team led by Prof. ZHOU Yongjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has achieved an advance in improving microbial production through lifespan engineering. Their findings, published in Proceedings of the National Academy of Sciences of the United States of America, demonstrate that combining lifespan engineering strategies with metabolic pathway optimization in Saccharomyces cerevisiae enables highly efficient sclareol biosynthesis.Building on the high-producing sclareol strain, the researchers systematically engineered cellular lifespan through four dimensions: nutrient sensing, mitophagy, protein stability, and genomic stability. Specifically, simultaneously weakening nutrient sensing and enhancing mitophagy, together with metabolic pathway optimization, resulted in a sclareol production of 25.9 g/L. Omics analysis showed that these strategies enhanced central metabolism and cellular robustness by extending chronological lifespan and regulating metabolic gene expression, thereby improving product synthesis during the later stages of cell growth.In addition, this lifespan engineering strategy also improved the biosynthesis of other products, such as sesquiterpenes and phenolic acids, indicating its broad applicability and providing a generalizable approach for developing high-performance microbial cell factories."Our work not only establishes a clear connection between chronological lifespan and biosynthesis capacity for improving sclareol production," said Prof. ZHOU, "but also offers a feasible longevity engineering strategy that can be extended to diverse microbial cell factories for sustainable and economical biomanufacturing."Lifespan engineering strategy (Image by WU Zulin and GAO Jiaoqi) -
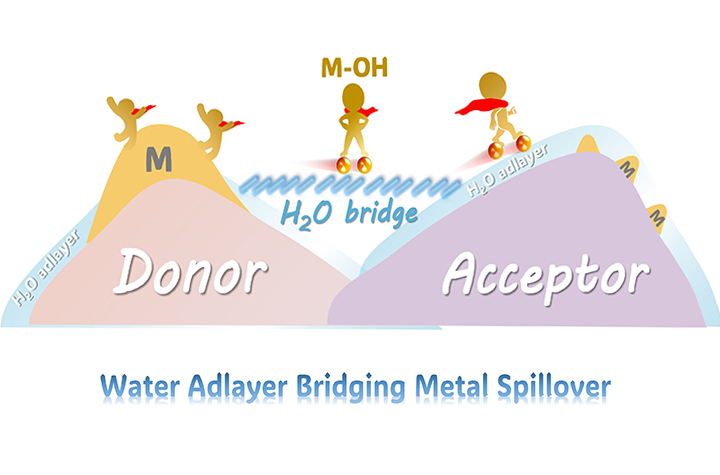 11 12, 2025Researchers Reveal Water-adlayer-mediated Metal Spillover Across Solid InterfacesProf. FU Qiang's team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has demonstrated a new form of metal spillover that is mediated by water adlayers.Hydrogen spillover is a well-established concept in heterogeneous catalysis where hydrogen atoms migrate across solid surfaces and interfaces. However, whether a similar process could occur for metal species has been an open question.In a study published in Nature Communications, a research team led by Prof. FU Qiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) demonstrated a new form of metal spillover that is mediated by water adlayers, and revealed that metal species can spontaneously migrate across solid interfaces under mild and humid conditions.Schematic illustration of water-adlayer-mediated metal spillover under mild conditions (Image by FAN Yamei)Researchers found that when hydrophilic supports such as oxides, carbides, or sulfides are exposed to a humid environment, a thin layer of water is formed on the surfaces. This water adlayer acts as a molecular bridge, enabling metal species, exemplified by copper, to migrate between different supports. The metal migration occurs through hydroxylated intermediates (M–OH) and proceeds spontaneously at room temperature without requiring high-temperature activation commonly used in catalyst preparation.Then, researchers showed that this spillover phenomenon is not limited to copper, and other metals including ruthenium, cobalt, and nickel also exhibit similar migration behavior. By tuning the surface hydrophilicity, the density of hydroxyl group, and the degree of interfacial contact, they were able to control metal mobility and distribution.Catalysts synthesized through this spillover route exhibited improved low-temperature activity in reactions, including carbon monoxide oxidation, reverse water–gas shift, ammonia selective catalytic reduction, and hydrogen cyanide oxidation. These catalysts outperformed those prepared using conventional impregnation methods.Mechanistic analysis showed that water adlayers and dissociated hydroxyl groups together form a hydration lubrication layer that lowers the diffusion barrier for metal atom migration. This mechanism is conceptually analogous to hydrogen spillover but represents the first direct extension of spillover to metal species. "Our study highlights how interfacial water layers and surface hydroxyls dynamically regulate catalyst structures and activities even under ambient conditions," said Prof. FU.This work not only provides a fundamental understanding of water-mediated metal migration, but also offers a new strategy for designing dynamic catalysts operable at low temperatures, promoting the development of energy-efficient catalytic systems.
11 12, 2025Researchers Reveal Water-adlayer-mediated Metal Spillover Across Solid InterfacesProf. FU Qiang's team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has demonstrated a new form of metal spillover that is mediated by water adlayers.Hydrogen spillover is a well-established concept in heterogeneous catalysis where hydrogen atoms migrate across solid surfaces and interfaces. However, whether a similar process could occur for metal species has been an open question.In a study published in Nature Communications, a research team led by Prof. FU Qiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) demonstrated a new form of metal spillover that is mediated by water adlayers, and revealed that metal species can spontaneously migrate across solid interfaces under mild and humid conditions.Schematic illustration of water-adlayer-mediated metal spillover under mild conditions (Image by FAN Yamei)Researchers found that when hydrophilic supports such as oxides, carbides, or sulfides are exposed to a humid environment, a thin layer of water is formed on the surfaces. This water adlayer acts as a molecular bridge, enabling metal species, exemplified by copper, to migrate between different supports. The metal migration occurs through hydroxylated intermediates (M–OH) and proceeds spontaneously at room temperature without requiring high-temperature activation commonly used in catalyst preparation.Then, researchers showed that this spillover phenomenon is not limited to copper, and other metals including ruthenium, cobalt, and nickel also exhibit similar migration behavior. By tuning the surface hydrophilicity, the density of hydroxyl group, and the degree of interfacial contact, they were able to control metal mobility and distribution.Catalysts synthesized through this spillover route exhibited improved low-temperature activity in reactions, including carbon monoxide oxidation, reverse water–gas shift, ammonia selective catalytic reduction, and hydrogen cyanide oxidation. These catalysts outperformed those prepared using conventional impregnation methods.Mechanistic analysis showed that water adlayers and dissociated hydroxyl groups together form a hydration lubrication layer that lowers the diffusion barrier for metal atom migration. This mechanism is conceptually analogous to hydrogen spillover but represents the first direct extension of spillover to metal species. "Our study highlights how interfacial water layers and surface hydroxyls dynamically regulate catalyst structures and activities even under ambient conditions," said Prof. FU.This work not only provides a fundamental understanding of water-mediated metal migration, but also offers a new strategy for designing dynamic catalysts operable at low temperatures, promoting the development of energy-efficient catalytic systems. -
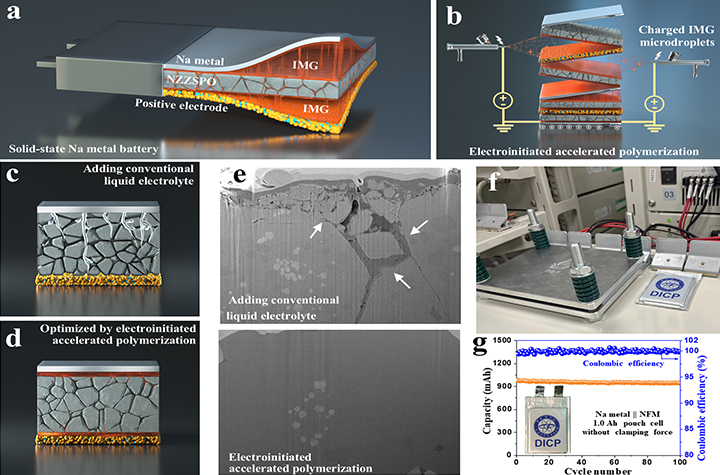 11 03, 2025Researchers Achieve External Pressure-free Solid-state Sodium Metal Batteries through New Interface Engineering ApproachA research team led by Prof. CHEN Zhongwei from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a new interfacial engineering strategy that enables external pressure-free solid-state sodium metal batteries, marking a major advance toward scalable and durable all-solid-state systems.Solid-state batteries have emerged as one of the most promising next-generation energy storage technologies due to their high energy density and intrinsic safety. However, their practical application remains hindered by poor interfacial contact and instability between the solid electrolyte and the metal anode.Interfacial modification strategy for Ah-level external pressure-free solid-state sodium metal batteries based on oxide solid-state electrolytes (Image by YANG Tingzhou)Recently, in a study published in Nature Communications, a research team led by Prof. CHEN Zhongwei from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a new interfacial engineering strategy that enables external pressure-free solid-state sodium metal batteries, marking a major advance toward scalable and durable all-solid-state systems.Among solid electrolytes, oxide-based electrolytes exhibit high ionic conductivity and chemical stability, but their intrinsic brittleness often leads to microcracks and pores during fabrication and operation. Moreover, poor wettability, high interfacial resistance, and side reactions during long-term cycling can further trigger dendrite penetration and interfacial failure. These issues severely hinder ion transport and battery reliability, making interfacial regulation and stabilization a central scientific challenge in solid-state battery development.To address these issues, the researchers proposed an innovative electrically induced accelerated polymerization strategy for interfacial repair. By introducing charged repair adhesive microdroplets, which undergo rapid polymerization under an electric field — 21.4 times faster than conventional methods — the process leverages the electrowetting effect to form a uniform interfacial coating that preferentially fills cracks and enhances interfacial contact.This strategy improves the stability between metallic sodium and the solid electrolyte, effectively preventing dendrite-induced crack propagation. As a result, the modified system achieved a critical current density of 6.8 mA cm-2 and sustained stable cycling over 1,000 cycles at 1.0 C.Furthermore, pouch-type solid-state sodium metal batteries constructed with this interfacial repair strategy maintained outstanding long-term cycling stability without external stack pressure, demonstrating its scalability and practical potential for next-generation energy storage.
11 03, 2025Researchers Achieve External Pressure-free Solid-state Sodium Metal Batteries through New Interface Engineering ApproachA research team led by Prof. CHEN Zhongwei from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a new interfacial engineering strategy that enables external pressure-free solid-state sodium metal batteries, marking a major advance toward scalable and durable all-solid-state systems.Solid-state batteries have emerged as one of the most promising next-generation energy storage technologies due to their high energy density and intrinsic safety. However, their practical application remains hindered by poor interfacial contact and instability between the solid electrolyte and the metal anode.Interfacial modification strategy for Ah-level external pressure-free solid-state sodium metal batteries based on oxide solid-state electrolytes (Image by YANG Tingzhou)Recently, in a study published in Nature Communications, a research team led by Prof. CHEN Zhongwei from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a new interfacial engineering strategy that enables external pressure-free solid-state sodium metal batteries, marking a major advance toward scalable and durable all-solid-state systems.Among solid electrolytes, oxide-based electrolytes exhibit high ionic conductivity and chemical stability, but their intrinsic brittleness often leads to microcracks and pores during fabrication and operation. Moreover, poor wettability, high interfacial resistance, and side reactions during long-term cycling can further trigger dendrite penetration and interfacial failure. These issues severely hinder ion transport and battery reliability, making interfacial regulation and stabilization a central scientific challenge in solid-state battery development.To address these issues, the researchers proposed an innovative electrically induced accelerated polymerization strategy for interfacial repair. By introducing charged repair adhesive microdroplets, which undergo rapid polymerization under an electric field — 21.4 times faster than conventional methods — the process leverages the electrowetting effect to form a uniform interfacial coating that preferentially fills cracks and enhances interfacial contact.This strategy improves the stability between metallic sodium and the solid electrolyte, effectively preventing dendrite-induced crack propagation. As a result, the modified system achieved a critical current density of 6.8 mA cm-2 and sustained stable cycling over 1,000 cycles at 1.0 C.Furthermore, pouch-type solid-state sodium metal batteries constructed with this interfacial repair strategy maintained outstanding long-term cycling stability without external stack pressure, demonstrating its scalability and practical potential for next-generation energy storage. -
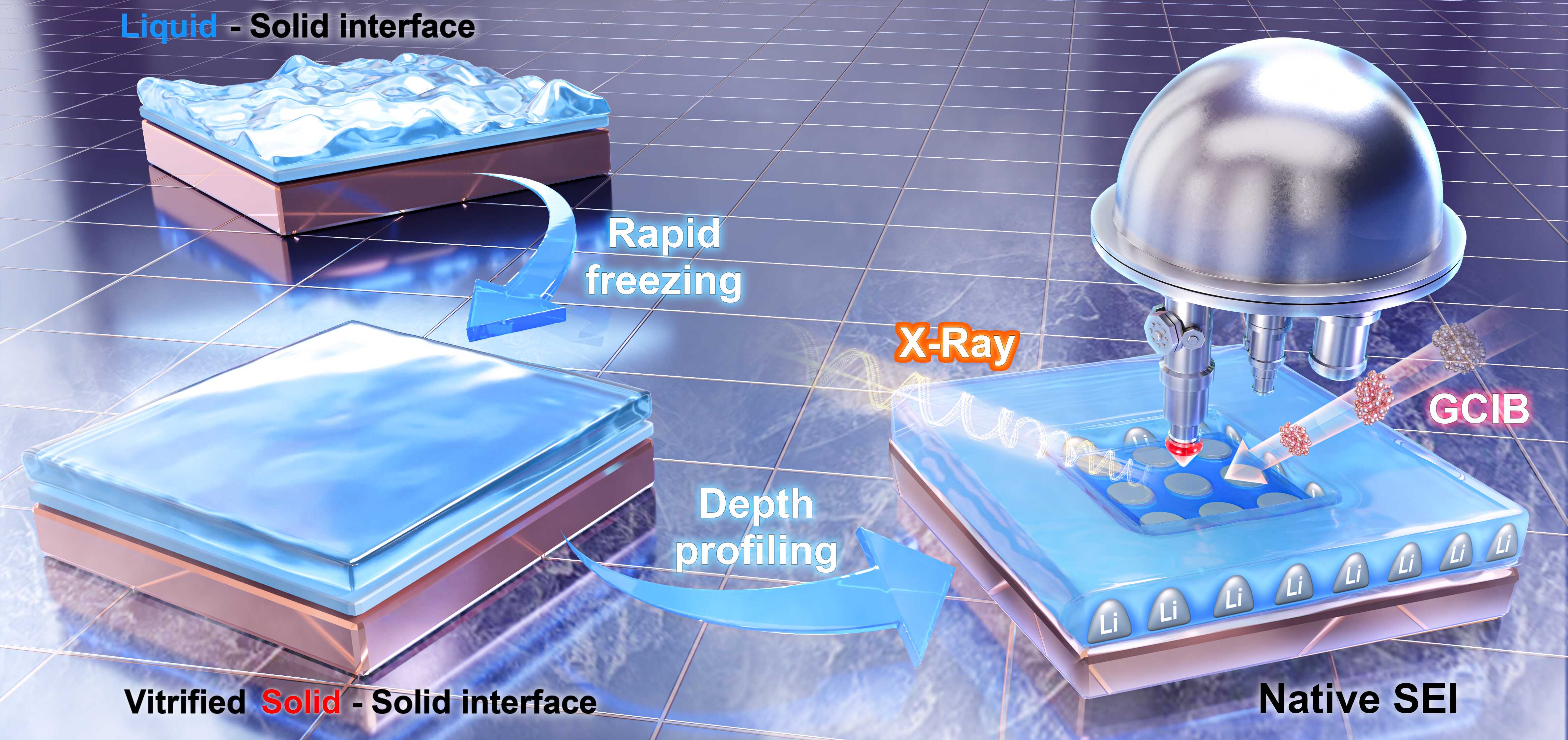 10 28, 2025Cryogenic X-ray Photoelectron Spectroscopy Reveals Native Solid Electrolyte Interphase Structure and Dynamics in Li Metal BatteriesBy coupling cryogenic X-ray photoelectron spectroscopy (cryo-XPS) with argon gas cluster ion beam (GCIB) sputtering, Prof. FU Qiang's team enables depth-resolved analysis of the vitrified solid electrolyte interphase under electrolyte conditions in Li metal batteries without inducing chemical damage.The solid electrolyte interphase (SEI) plays a pivotal role in governing the performance and stability of lithium (Li) batteries. X-ray photoelectron spectroscopy (XPS) is a powerful technique for identifying chemical states and distinguishing between organic and inorganic hybrid components within SEI architectures.However, conventional XPS techniques face two major challenges in characterizing native SEI structures. First, a fundamental incompatibility exists between the ultrahigh vacuum (UHV) environment required for XPS and the high saturated vapor pressures of organic liquid electrolytes used in Li metal batteries, leading to a critical "pressure gap". Second, conventional Ar⁺ sputtering often introduces chemical and structural artifacts, complicating accurate SEI analysis.Schematic diagram of the combined cryo-XPS and GCIB platform (Image by Shenghong Wang)In a recent study published in the Journal of the American Chemical Society, a research team led by Prof. FU Qiang and Dr. ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) addressed this challenge by coupling cryogenic X-ray photoelectron spectroscopy (cryo-XPS) with argon gas cluster ion beam (GCIB) sputtering. This integrated approach enables depth-resolved analysis of the vitrified SEI under electrolyte conditions ("wet"-SEI) in Li metal batteries without inducing chemical damage.The combined cryo-XPS and GCIB technique provides an unprecedented view of the native SEI composition in the presence of liquid electrolyte. The researchers revealed that the SEI consists of both organic polymeric hydrocarbons and inorganic species such as LiCx, LiF, LiOx, and Li2CO3. These results differ from conventional XPS analyses of "dry"-SEI layers, which typically show inorganic species due to artifact formation. The findings highlight the strength of cryo-XPS and GCIB approach in revealing the true structure and chemistry of the native SEI.Furthermore, the researchers identified a graded SEI architecture: electrochemical decomposition products such as LiF and Li2CO3 dominate the electrolyte-facing regions, while chemically derived species such as LiOx and LiCx accumulate near the electrode interface.In addition, the researchers also tracked the evolution of SEI during Li deposition, unraveling a compositional shift from an electrochemical SEI to a graded, complex SEI architecture accompanied by a thickness increase from nanometer to micrometer scale."Our study introduces a promising methodology for elucidating dynamic and heterogeneous chemical signatures across evolving solid-liquid interfaces in electrocatalysis and energy storage systems," said Prof. FU.
10 28, 2025Cryogenic X-ray Photoelectron Spectroscopy Reveals Native Solid Electrolyte Interphase Structure and Dynamics in Li Metal BatteriesBy coupling cryogenic X-ray photoelectron spectroscopy (cryo-XPS) with argon gas cluster ion beam (GCIB) sputtering, Prof. FU Qiang's team enables depth-resolved analysis of the vitrified solid electrolyte interphase under electrolyte conditions in Li metal batteries without inducing chemical damage.The solid electrolyte interphase (SEI) plays a pivotal role in governing the performance and stability of lithium (Li) batteries. X-ray photoelectron spectroscopy (XPS) is a powerful technique for identifying chemical states and distinguishing between organic and inorganic hybrid components within SEI architectures.However, conventional XPS techniques face two major challenges in characterizing native SEI structures. First, a fundamental incompatibility exists between the ultrahigh vacuum (UHV) environment required for XPS and the high saturated vapor pressures of organic liquid electrolytes used in Li metal batteries, leading to a critical "pressure gap". Second, conventional Ar⁺ sputtering often introduces chemical and structural artifacts, complicating accurate SEI analysis.Schematic diagram of the combined cryo-XPS and GCIB platform (Image by Shenghong Wang)In a recent study published in the Journal of the American Chemical Society, a research team led by Prof. FU Qiang and Dr. ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) addressed this challenge by coupling cryogenic X-ray photoelectron spectroscopy (cryo-XPS) with argon gas cluster ion beam (GCIB) sputtering. This integrated approach enables depth-resolved analysis of the vitrified SEI under electrolyte conditions ("wet"-SEI) in Li metal batteries without inducing chemical damage.The combined cryo-XPS and GCIB technique provides an unprecedented view of the native SEI composition in the presence of liquid electrolyte. The researchers revealed that the SEI consists of both organic polymeric hydrocarbons and inorganic species such as LiCx, LiF, LiOx, and Li2CO3. These results differ from conventional XPS analyses of "dry"-SEI layers, which typically show inorganic species due to artifact formation. The findings highlight the strength of cryo-XPS and GCIB approach in revealing the true structure and chemistry of the native SEI.Furthermore, the researchers identified a graded SEI architecture: electrochemical decomposition products such as LiF and Li2CO3 dominate the electrolyte-facing regions, while chemically derived species such as LiOx and LiCx accumulate near the electrode interface.In addition, the researchers also tracked the evolution of SEI during Li deposition, unraveling a compositional shift from an electrochemical SEI to a graded, complex SEI architecture accompanied by a thickness increase from nanometer to micrometer scale."Our study introduces a promising methodology for elucidating dynamic and heterogeneous chemical signatures across evolving solid-liquid interfaces in electrocatalysis and energy storage systems," said Prof. FU.