Sugar is a common dietary component, yet excessive intake has well-documented negative health impacts. Steviol glycosides, natural sweeteners extracted from Stevia rebaudiana, are widely used as sucrose substitutes due to their high sweetness and low caloric value. Among them, Rebaudioside M (Reb M) is regarded as a next-generation, high-value steviol glycoside product because of its intense sweetness and superior taste profile. However, the natural abundance of Reb M in stevia is extremely low, creating the need for efficient biosynthetic methods to meet market demand.
Until now, the key enzyme catalyzing the conversion of Rebaudioside D (Reb D) to Reb M in the biosynthetic pathway had not been clearly identified and was generally assumed to be UGT76G1. However, UGT76G1 exhibits strict regioselectivity for the C13 position of steviol glycosides, while its catalytic activity at the C19 position is very weak.
In a recent study published in Proceedings of the National Academy of Sciences, a team led by Prof. YIN Heng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) identified the key glycosyltransferase that catalyzes the conversion of Reb D to Reb M and revealed the molecular mechanism underlying its substrate regioselectivity. By applying semi-rational design, the team further engineered several tool enzymes with enhanced performance, enabling the efficient synthesis of Reb M.
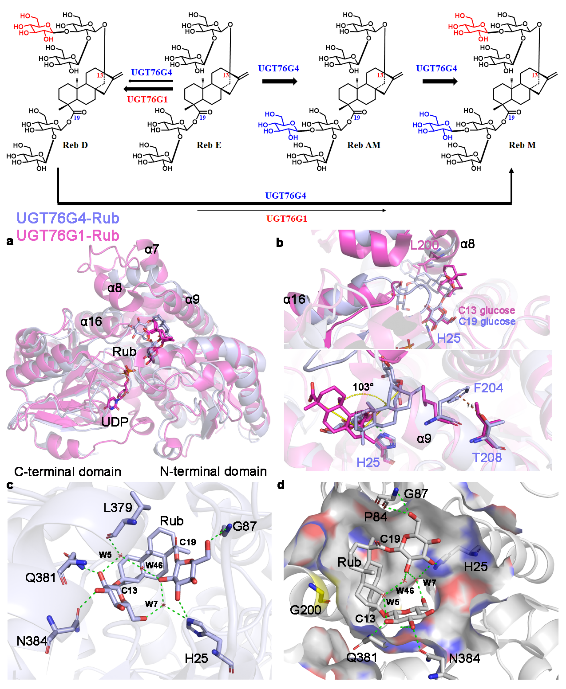
Key role of UGT76G4 in Rebaudioside M synthesis and the structural basis of its substrate regioselectivity. (Image by WANG Yu and LI Tang)
Researchers identified and characterized UGT76G4—a natural variant of UGT76G1—from a high-Reb M producing Stevia cultivar. They discovered that UGT76G4 exhibited regioselectivity toward the C19 position of steviol glycosides in both in vivo and in vitro assays, thereby confirming it as the key enzyme catalyzing the conversion of Reb D to Reb M.
Furthermore, researchers systematically elucidated the molecular basis of UGT76G4 regioselectivity, identifying residue G200 as critical for its C19 catalytic activity. Based on this mechanistic insight, they engineered UGT76G4 variants capable of efficiently synthesizing Reb M using Rebaudioside E (Reb E) and Reb D as substrates.
"This work not only clarifies the long standing question of which enzyme is responsible for Reb M biosynthesis, but also provides engineered biocatalysts to enable the efficient production of this next generation natural sweetener," said Prof. YIN.