Hydroformylation, a key homogeneous reaction known for its atom economy, is a crucial industrial process for converting alkenes and syngas to aldehydes. These aldehydes can be used in the production of alcohols, amines, carboxylic acids, and other high-value-added fine chemicals. Currently, the annual production of aldehydes and their subsequent hydrogenated alcohols through hydroformylation exceeds 25 million tons.
A research group led by Prof. YAN Li and Prof. DING Yunjie from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Science (CAS) developed a highly active and stable porous organic polymer supported single rhodium site (Rh-POPs) catalyst. This catalyst was applied to heterogeneous ethylene hydroformylation, addressing the challenges of precious metal and phosphorus ligand loss in traditional homogeneous technologies.
In August 2020, the world's first demonstration of heterogeneous ethylene hydroformylation and subsequent hydrogenation to produce n-propanol (50000 t/year) using the Rh-POPs catalysts was completed in Zhejiang Province, China. The plant has been operating steadily for the past four years.
Recently, this group further investigated and revealed the dynamic mechanism of tunable regioselectivity in propylene hydroformylation. Their study was published in Nature Communications.
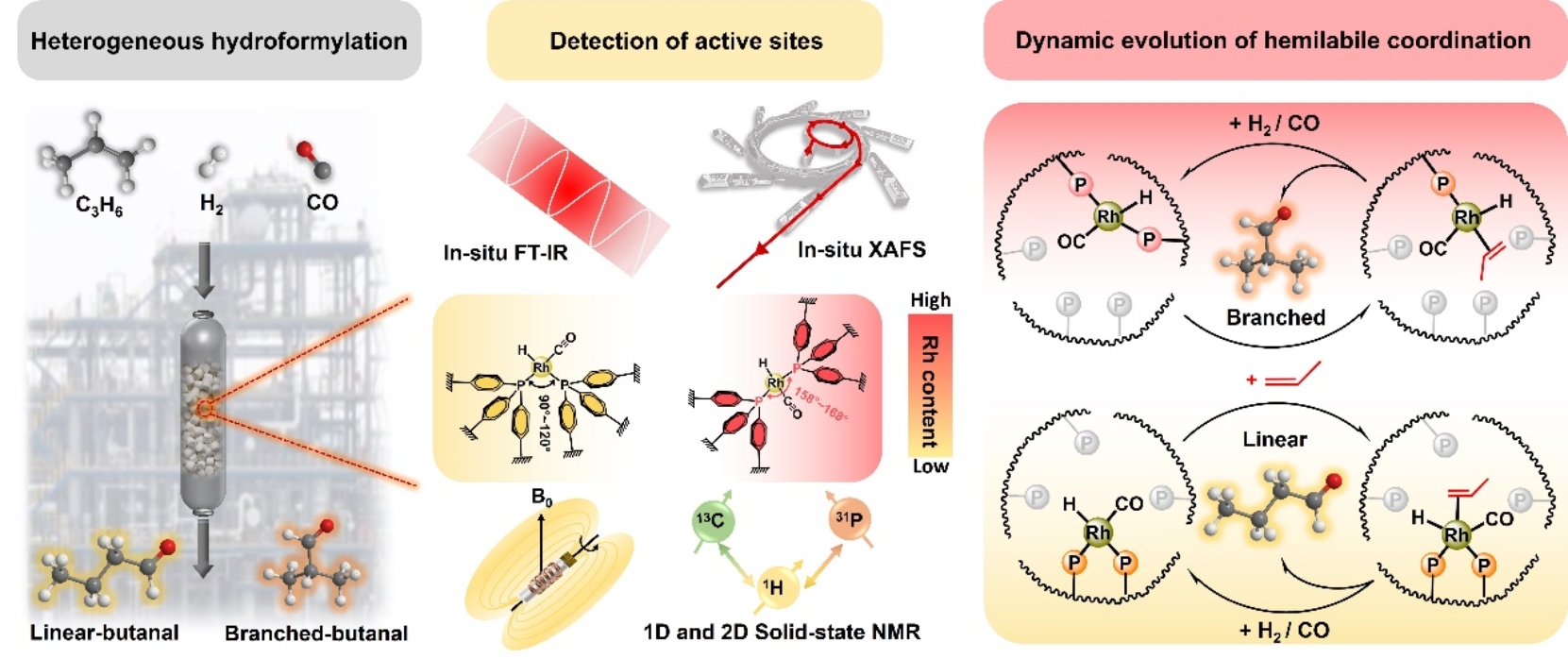
Schemes to map the active center and the dynamic evolution of hemilabile coordination (Image by FAN Benhan)
The researchers studied the precise structure of the active centers in industrially applied Rh-POPs catalysts and their dynamic evolution under real reaction conditions. They found that during the hydroformylation, the adsorption, activation, reaction, and desorption of guest molecules on the Rh-P coordination active centers were accompanied by the dissociation and re-coordination of Rh-P hemilabile-coordination bonds. This process enabled the Rh-P active centers to dynamically shift between "open" and "closed" coordination states, forming different steric hindrances that regulated the regioselectivity of the aldehyde product.
“These state-of-the-art techniques and multiscale analysis help us to understand how the hemilabile effect in heterogeneous catalysis influences the electronic state and geometric configuration of metal active centers, further affecting the catalyst's activity and regioselectivity,” said Prof. YAN
“It provides novel insights for the rational design of hydroformylation catalysts with tunable regioselectivity for future industrial applications,” said Prof. DING.