The solid electrolyte interphase (SEI) plays a pivotal role in governing the performance and stability of lithium (Li) batteries. X-ray photoelectron spectroscopy (XPS) is a powerful technique for identifying chemical states and distinguishing between organic and inorganic hybrid components within SEI architectures.
However, conventional XPS techniques face two major challenges in characterizing native SEI structures. First, a fundamental incompatibility exists between the ultrahigh vacuum (UHV) environment required for XPS and the high saturated vapor pressures of organic liquid electrolytes used in Li metal batteries, leading to a critical "pressure gap". Second, conventional Ar⁺ sputtering often introduces chemical and structural artifacts, complicating accurate SEI analysis.
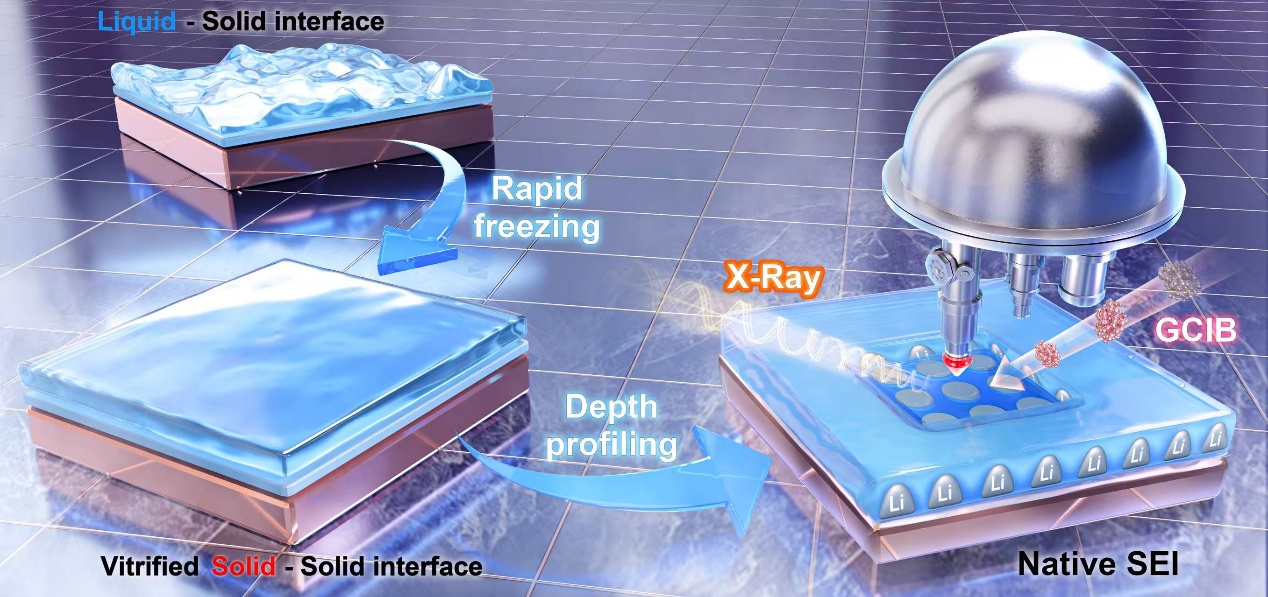
Schematic diagram of the combined cryo-XPS and GCIB platform (Image by Shenghong Wang)
In a recent study published in the Journal of the American Chemical Society, a research team led by Prof. FU Qiang and Dr. ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) addressed this challenge by coupling cryogenic X-ray photoelectron spectroscopy (cryo-XPS) with argon gas cluster ion beam (GCIB) sputtering. This integrated approach enables depth-resolved analysis of the vitrified SEI under electrolyte conditions ("wet"-SEI) in Li metal batteries without inducing chemical damage.
The combined cryo-XPS and GCIB technique provides an unprecedented view of the native SEI composition in the presence of liquid electrolyte. The researchers revealed that the SEI consists of both organic polymeric hydrocarbons and inorganic species such as LiCx, LiF, LiOx, and Li2CO3. These results differ from conventional XPS analyses of "dry"-SEI layers, which typically show inorganic species due to artifact formation. The findings highlight the strength of cryo-XPS and GCIB approach in revealing the true structure and chemistry of the native SEI.
Furthermore, the researchers identified a graded SEI architecture: electrochemical decomposition products such as LiF and Li2CO3 dominate the electrolyte-facing regions, while chemically derived species such as LiOx and LiCx accumulate near the electrode interface.
In addition, the researchers also tracked the evolution of SEI during Li deposition, unraveling a compositional shift from an electrochemical SEI to a graded, complex SEI architecture accompanied by a thickness increase from nanometer to micrometer scale.
"Our study introduces a promising methodology for elucidating dynamic and heterogeneous chemical signatures across evolving solid-liquid interfaces in electrocatalysis and energy storage systems," said Prof. FU.