Lithium (Li) metal is considered as the "Holy Grail" among the anode materials, due to its high theoretical capacity density and low electrochemical potential.
However, the cycle life of lithium metal batteries is normally limited by the repeated rupture of solid electrolyte interphase (SEI) that is always too brittle to withstand severe electrode interface deformation during cycling.
Recently, a research group led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a paradigmatic N-rich polyether additive, nitrocellulose (NC), to stabilize the electrolytes for Li metal batteries.
This study was published in Angewandte Chemie International Edition on March 10.
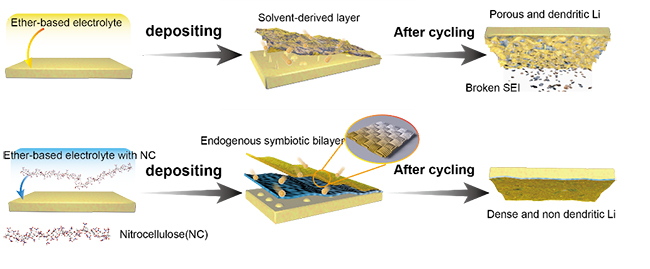
Illustration of the endogenous symbiotic Li3N/cellulose double SEI using nitrocellulose (Image by LUO Yang)
"We investigated the underlying reaction mechanism of lithium and NC by combing the detailed experimental characterizations and density functional theory (DFT) calculations," said Prof. LI.
The researchers found that the nitro group of NC could react with lithium metal, and the cellulose skeleton was tightly wrapped on the surface via Li-O bond. Therefore, the endogenous symbiotic Li3N/cellulose double SEI (ES-DSEI) was formed on the lithium surface almost simultaneously.
Moreover, they confirmed the structure and composition of the as-obtained bilayer by cryo-environmental transmission electron microscopy (ETEM) and X-ray photoelectron spectroscopy (XPS).
Lithium anode presented a dendrite-free morphology, which was detected by on-site optical microscopy and prolonged its cycle life due to the ES-DSEI. And the lithium metals with ES-DSEI were promising to be used in practical applications especially at elevated temperatures.
This work provides new insights on the exact role of additives at a fundamental level.
This work was supported by the National Natural Science Foundation of China and Youth Innovation Promotion Association of CAS. (Text by LUO Yang)