Research News
-
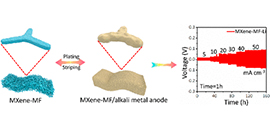 07 28, 2020Scientists Develops 3D MXene-Melamine Foam for High-areal-capacity and Long-lifetime Alkali-metal AnodesScientists from DICP and etc developed a recyclable, flexible and conductive 3D porous MXene-MF aerogel, for dendrite-free, stable, high capacity alkali-metal anodes.
07 28, 2020Scientists Develops 3D MXene-Melamine Foam for High-areal-capacity and Long-lifetime Alkali-metal AnodesScientists from DICP and etc developed a recyclable, flexible and conductive 3D porous MXene-MF aerogel, for dendrite-free, stable, high capacity alkali-metal anodes.
Alkali-metal anodes are considered as ideal anodes for high-energy-density batteries due to their high theoretical capacities and low redox potential.
However, the undesirable growth of dendrite and infinite relative change of volume during cycling processes have blocked the commercialization of alkali-metal anodes.
Researchers led by Prof. WU Zhongshuai in collaboration with Prof. LI Xianfeng, Dr. ZHENG Qiong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, and Prof. YU Yan from University of Science and Technology of China, developed flexible three-dimensional (3D) MXene/Melamine foam (MXene-MF) with high electronic conductivity for alkali-metal anode with ultrahigh current density and capacities.
The study was published in ACS Nano on June 12.
The scientists took advantages of high conductivity and lithiophilic surfaces from MXene, in combination of porous, lightweight and flexible nature from 3D MF to develop the MXene-MF.
It possessed 3D interconnected porous conductive network and showed good mechanical flexibility and strength, enabling a highly dense and homogenous alkali-metal deposition and suppressing volume fluctuation at both high current density and deposition capacity. Consequently, it achieved a high current density of 50 mA/cm2 for 50 mAh/cm2 for Li anode, and realized a high areal deposition capacity of 20 mAh/cm2 for Na anode.
Theoretical simulation demonstrated that the MXene-MF electrode could significantly reduce current density and guide the lithium ions deposition on the MXene skeleton. Moreover, such a robust 3D architecture guaranteed the repeated cycling without decomposing the structural integrity.
By pairing MXene-MF-Li with sulfur and MXene-MF-Na with Na3V2(PO4)3, respectively, the assembled full-batteries showed improved cycling and rate capability. MXene-MF is promising in constructing high-energy-density batteries.
Schematic of 3D MXene-MF foam for high current-density, high-capacity alkali-metal anodes (Image by SHI Haodong and HOU Xiaocheng)
The study was supplied by National Natural Science Foundation of China, National Key R&D Program of China, Dalian National Laboratory For Clean Energy (DNL), CAS. (Text by SHI Haodong and HOU Xiaocheng) -
 07 20, 2020Scientists Develop Novel MoS2 Nanofoam Catalyst to boost Hydrogen Evolution ReactionScientists from DICP reported a MoS2 nanofoam catalyst with co-confined Se in the surface and Co in the inner layer of the tri-atomic-layer structure of MoS2, which exhibited high catalytic activity and durability for acidic HER.
07 20, 2020Scientists Develop Novel MoS2 Nanofoam Catalyst to boost Hydrogen Evolution ReactionScientists from DICP reported a MoS2 nanofoam catalyst with co-confined Se in the surface and Co in the inner layer of the tri-atomic-layer structure of MoS2, which exhibited high catalytic activity and durability for acidic HER.
Two-dimensional (2D) MoS2 has shown good catalytic activity for the hydrogen evolution reaction (HER) and is considered as a potential alternative to the precious platinum-based catalysts in acidic medium.
However, only the edge S sites of pure MoS2 are catalytically active for HER and the vast amount of S sites in the basal plane are quite inert and not sufficiently utilized. Besides, the low stability of edges also limits HER performance of MoS2.
Therefore, selectively activating the inert basal plane combined with enriching and stabilizing edges can increase active sites of MoS2 for HER. It is still highly challenging owing to the difficulty in balancing activity and stability.
Recently, Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences reported a MoS2 nanofoam catalyst with co-confined Se in the surface and Co in the inner layer of the tri-atomic-layer structure of MoS2. It exhibited high catalytic activity and durability for acidic HER at a large current density of 1000 mA/cm2.
The study was published in Nature Communications on July 3. It opens up a new prospect of tailoring the catalytic performance of MoS2 toward large-scale HER applications through confining multi-elements in different atomic layers.
High-performance hydrogen evolution reaction over the Co/Se-codoped MoS2 (Image by ZHENG Zhilong, YU Liang and GAO Hehua)
The scientists previously reported a strategy of confining transition metal atoms into MoS2 lattice to trigger HER activity over S atoms in the inert basal plane.
In this study, they found that co-confining Se in the surface and Co in the inner layer of the MoS2 could realize simultaneously activation of the basal plane, optimization of the hydrogen adsorption activity, and stabilization of the structure.
"Such catalyst exhibited an ultra-high HER activity in acidic medium" said Prof. DENG.
They achieved a much lower overpotential of 382 mV than that of 671 mV over commercial Pt/C catalyst at a large current density of 1000 mA/cm2, and it stably maintained for 360 hours without decay. The activity surpasses those of all previously reported heteroatom-doped MoS2.
Density functional theory calculations demonstrated that inner layer-confined Co atoms stimulated neighbouring S atoms while surface-confined Se atoms stabilized the structure, which cooperatively enabled the massive generation of both in-plane and edge active sites with optimized hydrogen adsorption activity.
The study was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund, CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text by ZHENG Zhilong, YU Liang and GAO Hehua) -
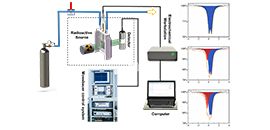 07 13, 2020Scientists Develop In-situ/Operando Electrochemical 57Fe and 119Sn Mössbauer InstrumentScientists from DICP dependently developed a set of in-situ/operando electrochemical 57Fe and 119Sn M?ssbauer instrument
07 13, 2020Scientists Develop In-situ/Operando Electrochemical 57Fe and 119Sn Mössbauer InstrumentScientists from DICP dependently developed a set of in-situ/operando electrochemical 57Fe and 119Sn M?ssbauer instrument
Mossbauer spectroscopy is the science of studying the behavior of electrons outside the nucleus. It has high energy resolution and can observe the hyperfine interactions of Mossbauer nuclides, and characterize the phase, valence state, coordination structure and magnetism of the Mossbauer elements in samples, such as iron and tin.
The in situ/operando electrochemical Mossbauer spectroscopy can be used to explore the changes of electronic, structural and magnetic properties of the electrocatalytic materials.It' a powerful tool to study the structure-activity relationship and mechanism of electrocatalyst in electrochemical reaction.
Recently, Prof. WANG Junhu's Group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed a set of in-situ/operando electrochemical 57Fe and 119Sn Mossbauer instrument. It can be used to dynamically observe the evolution of active phases of iron-based and tin-based catalysts in electrochemical reactions, and reveal the intrinsic nature of the electrocatalysts.
In this instrument, single line Mossbauer radiation source used keV level gamma ray, which could reduce attenuation in electrolyte and obtain satisfied signal-to-noise ratio.
Electrocatalyst sample only needs to be evenly coated on carbon paper or carbon cloth before the test, and then the electrochemistry and Mossbauer spectroscopy could be simultaneously conducted.
The thickness of the in-situ cell was 5 mm, which could well consider the exposure and detection efficiency of the active phase of the catalyst. The radioactive source, sample cell and detector were assembled horizontally. The in-situ/operando system was easy to be installed and disassembled flexibly.
Schematically show the developed in-situ/operando electrochemical 57Fe and 119Sn Mossbauer instrument and practical example of three spectra of the topotactically constructed nickel-iron (oxy)hydroxide electrocatalyst with abundant Fe4+ species generated during water oxidation rection (Image by KUANG Zhichong)
This instrument had been applied in the oxygen evolution reaction, oxygen reduction reaction and carbon dioxide reduction reaction. The test results showed that the obtained spectra had satisfied signal-to-noise ratio.
For the in-situ/operando testing, samples may be prepared using enriched isotopes 57Fe or 119Sn if the iron or tin content of the sample is too small.
This instrument provides a high level of research tool for the preparation of highly efficient catalysts of electrolytic water and carbon dioxide reduction. (Text by KUANG Zhichong) -
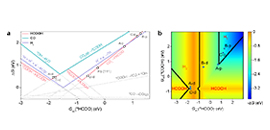 07 10, 2020New Approach to Study CO2 Electroreduction Reaction Mechanism and Active SiteScientists proposed a new approach to study the reaction mechanism and active site for electrochemical CO2RR via reaction phase diagram.
07 10, 2020New Approach to Study CO2 Electroreduction Reaction Mechanism and Active SiteScientists proposed a new approach to study the reaction mechanism and active site for electrochemical CO2RR via reaction phase diagram.
Electrochemical CO2 reduction reaction (CO2RR), driven by the sustainable electric energies, can convert the carbon dioxide into valuable chemicals and fuels.
Scientists prepared a hydrocerussite [Pb3(CO3)2(OH)2] as the electrocatalyst of CO2RR, which increased the Faradaic efficiency to 96% for formate production. However, the reaction mechanism and active site of it remain unclear.
Recently, a research group led by Prof. XIAO Jianping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in cooperation with Prof. ZHANG Bin from Tianjin University, proposed a new approach to study the reaction mechanism and active site for electrochemical CO2RR via reaction phase diagram.
Reaction phase diagram (a, b), free energy diagram (c) and liner correlation between the experimantal current density and theoretical charge transfer rate (d) (Image by LONG Jun)
The scientists conducted density functional theory calculations to understand the reaction mechanism and active site.
According to the bulk structure of hydrocerussite, three terminations were possible to be exposed. For each termination, the vacancy defect of CO3- or OH- might be formed since hydrocerussite was in situ produced. Therefore, six different reaction sites could be obtained.
The scientists found that the adsorption energies of involved intermediates had good correlation on these sites, based on which the reaction phase diagram was constructed with the adsorption energy of *HCOO as the descriptor. They observed two activity volcanos for formate production because the reaction could follow either a *HCOO or *COOH mechanism.
Compared with experimental results, the vacancy site of termination B was determined as the active site, which showed the high activity and selectivity simultaneously.
Besides, the charge transfer rate over this site was calculated by microkinetic simulation, which exhibited a nice linear correlation with the experimental formate partial current density, confirming the above calculations and analysis.
The descriptors-based method can also be generalized to other reaction systems, providing a new way to study the reaction mechanism and the product selectivity of active sites.
This study was published in Nature Communications. It was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of Chinese Academy of Sciences and Liaoning Revitalization Talents program. (Text by LONG Jun) -
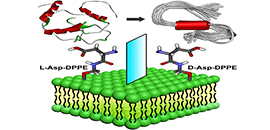 07 09, 2020New Findings Shed Light on Development of Liposome-based InhibitorsScientists from DICP designed and prepared a pair of chiral amino acid?modified phospholipids, and displayed the remarkable influence of molecular chirality of chiral liposomes on the amyloid formation.
07 09, 2020New Findings Shed Light on Development of Liposome-based InhibitorsScientists from DICP designed and prepared a pair of chiral amino acid?modified phospholipids, and displayed the remarkable influence of molecular chirality of chiral liposomes on the amyloid formation.
Alzheimer's disease (AD) is one of the biggest global public health challenges. However, the pathogenesis of AD is still unclear.
A number of studies showed that cell membranes play crucial roles in the progress of AD, particularly amyloid-β (Aβ) accumulation. Therefore it's essential to investigate the effect of biological membranes on amyloid formation.
Molecular chirality mediated amyloid formation on phospholipid surfaces (Image by WANG Xue)
Recently, research groups led by Prof. QING Guangyan and Prof. LI Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences designed and prepared a pair of chiral amino acid-modified phospholipids, showing remarkable influence of molecular chirality of chiral liposomes on the amyloid formation.
The researchers found that the self-assembled L-liposomes slightly inhibited Aβ(1-40) nucleation process but could not affect oligomer elongation process. By comparison, the D-liposomes strongly inhibited both the nucleation and elongation processes of Aβ(1-40).
Chiral liposomes not only had good biocompatibility but also could rescue Aβ(1-40) aggregation induced cytotoxicity with significant chiral discrimination, in which the cell viability was higher in the presence of D-liposomes.
Meanwhile, The scientists revealed the binding site, binding manners and driving force between Aβ(1-40) and chiral phospholipid surfaces through detailed molecular dynamics simulations.
These findings expanded the research from artificial chiral surfaces to real chiral phospholipid surfaces, providing a deeper and real insight to understand the crucial amyloidosis process from the perspective of chiral biointerface.
Liposomes have convincing biocompatibility, and the convergence of liposomes with non-natural D-amino acids as amyloid inhibitors have great application prospects in the AD early prevention and treatment, which points out a clear direction for the development of liposome-based inhibitors.
The study was published in Chemical Science. It was supported by the National Natural Science Foundation of China, the Start-up Fund of the Innovation Special Zone Group of DICP, and the Xingliao Elite Program. (Text by WANG Xue) -
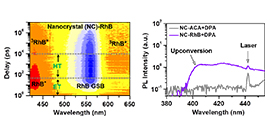 07 08, 2020Scientists Reveal New Mechanism for Triplet Energy Migration Across Inorganic/Organic InterfaceScientists from DICP observed the mechanisms for triplet energy migration across the inorganic/organic interface. They revealed a new mechanism called electron transfer-mediated triplet energy migration.
07 08, 2020Scientists Reveal New Mechanism for Triplet Energy Migration Across Inorganic/Organic InterfaceScientists from DICP observed the mechanisms for triplet energy migration across the inorganic/organic interface. They revealed a new mechanism called electron transfer-mediated triplet energy migration.
Triplet energy transfer from colloidal nanocrystals is a novel approach to sensitizing molecular triplets. Recent studies suggest that triplet transfer can be mediated by a hole transfer process when it is energetically allowed.
However, electron-transfer-mediated triplet transfer has not been observed yet. It is probably due to that the hole-trapping in typical II-VI group nanocrystals inhibits the hole transfer step and the following initial electron transfer, hence disrupting a complete triplet exciton transfer.
Recently, a research group led by Prof. WU Kaifeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) observed the mechanisms for triplet energy migration across the inorganic/organic interface. They revealed a new mechanism called electron transfer-mediated triplet energy migration.
The results were published in J. Am. Chem. Soc. on June 1.
Electron transfer-mediated triplet energy migration from semiconductor nanocrystals to surface-anchored molecular acceptors(Image by LUO Xiao)
The scientists observed electron-transfer-mediated triplet energy transfer from CsPbCl3 and CsPbBr3 perovskite nanocrystals to surface-anchored Rhodamine molecules, and establised the mechanism by ultrafast spectroscopy.
The control experiments using CdS nanocrystals also confirmed the role of hole-trapping in inhibiting this mechanism.
The sensitized Rhodamine triplets engaged in a variety of applications such as photon upconversion and singlet oxygen generation.
“Compared to conventional one-step triplet transfer, this new sensitization mechanism is less demanding in terms of interfacial electronic coupling and hence is more generally implementable” said prof. WU.
This study not only establishes a complete framework of triplet energy transfer across nanocrystal/molecule interfaces, but also greatly expands the scope of molecular triplet sensitization using nanocrystals.
The research was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Sciences, and the LiaoNing Revitalization Talents Program. (Text by LUO Xiao).