Converting CO2 to value-added chemicals driven by renewable energy is a strategy of reducing carbon emission and alleviating pressure from depleting fossil resources. Electrocatalytic CO2 reduction (CO2R) to CO is an important way for CO2 utilization since CO is widely used for chemical synthesis.
Metal-free nitrogen-doped (N-doped) carbon material possesses high conductivity and tunable electronic structure. It exhibits excellent performance for the electrocatalytic CO2R to CO. However, the active site for the reaction remains under debate due to the complexity in the N species as well as the lack of methods for controllable doping of N.
Recently, a research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) unveiled the nature of the active site in N-doped carbon materials for the electrocatalytic CO2R to CO. The study was published in Cell Reports Physical Science on August 12.
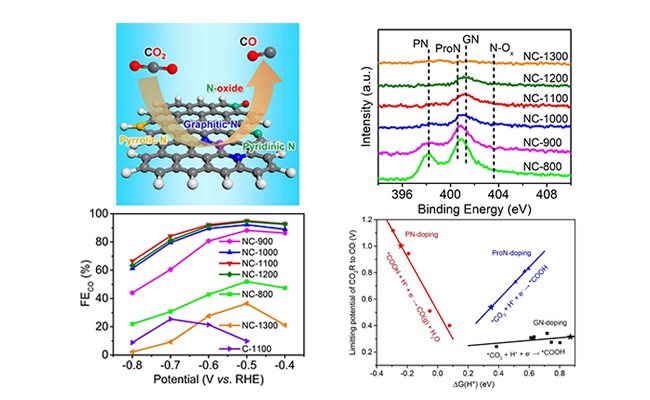
Electrocatalytic performances of N-doped carbon foam on the CO2R to CO reaction (Image by (Text by ZHANG Zheng, YU Liang, and GAO Hehua)
Via an innovated approach of silicon dioxide nanosphere template-assisted pyrolysis of phthalocyanine, the scientists prepared a series of N-doped carbon foam catalysts with precisely controlled N-dopant types and contents.
Electrochemical tests showed that at -0.5 V versus reversible hydrogen electrode (RHE), the catalyst dominated by graphitic N (GN) dopants exhibited the highest CO Faradaic efficiency of 95%, compared with those dominated by pyridinic N (PN) or pyrrolic N (ProN) dopants.
Theoretical calculations demonstrated that the limiting potentials for CO2R to CO over the carbon atoms adjacent to the GN were significantly lower than those for the hydrogen evolution reaction (HER), which favored the electrocatalytic CO2R.
The PN site tended to be blocked by the strongly adsorbed H* and the carbon atoms neighbouring to the PN possessed lower limiting potentials for HER, while the ProN disfavored both CO2R and HER.
"Compared with the PN and ProN, the GN significantly improved the activity of the adjacent carbon atoms for the electrocatalytic CO2R to CO," said Prof. DENG.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of Chinese Academy of Sciences, the DNL Cooperation Fund, CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011.iChEM). (Text by ZHANG Zheng, YU Liang, and GAO Hehua)