A research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences innovated the preparation strategy to construct robust interface Ru centers between RuO2 and graphene. It enhances both activity and stability of the acidic oxygen evolution reaction (OER), which provides a new strategy of designing highly active and stable OER catalyst.
As a clean, efficient and renewable green energy source, hydrogen is regarded as the most potential energy in the 21st century. Acidic water electrolysis based on the proton exchange membrane with the characteristics of high efficiency, high hydrogen production purity and low energy consumption, is considered as one of the most promising technologies for continuous production of high-purity hydrogen.
The anodic oxygen evolution reaction has become the bottleneck of water electrolysis due to its dynamic inertness and four-electron transfer process. So far, RuO2 is one of the very few catalysts that can catalyze the OER in acidic electrolyte. However, the accompanying over-oxidation of RuO2 to dissolvable RuO4 at applied OER potentials together with the acidic environment make RuO2 suffer from strong corrosion and therefore unable to sustain the OER, which seriously prevents its application in acidic electrolyte.
Although many efforts have been devoted to enhancing the OER performance in acidic electrolyte, it is still a great challenge to concurrently achieve highly active and long-term stable catalysts up to date.
Scientists innovated the preparation strategy to construct robust interface Ru centers between RuO2 and graphene via a controllable oxidation of graphene encapsulating Ru nanoparticles to efficiently enhance both activity and stability of the acidic OER.
Through precisely controlling the reaction interface, a much lower OER overpotential of only 227 mV at 10 mA/cm2 in acidic electrolyte compared with that of 290 mV for commercial RuO2, but a significantly higher durability than the commercial RuO2 were achieved. Besides, cooperating with Dr. REN Pengju from Institute of Coal Chemistry, Chinese Academy of Sciences on density functional theory calculations, they demonstrated that the interface Ru centers between RuO2 and graphene can break the classic scaling relationships between the free energies of HOO* and HO* to reduce the limiting potential, rendering an enhancement in the intrinsic OER activity and the resistance to over-oxidation and corrosion for RuO2.
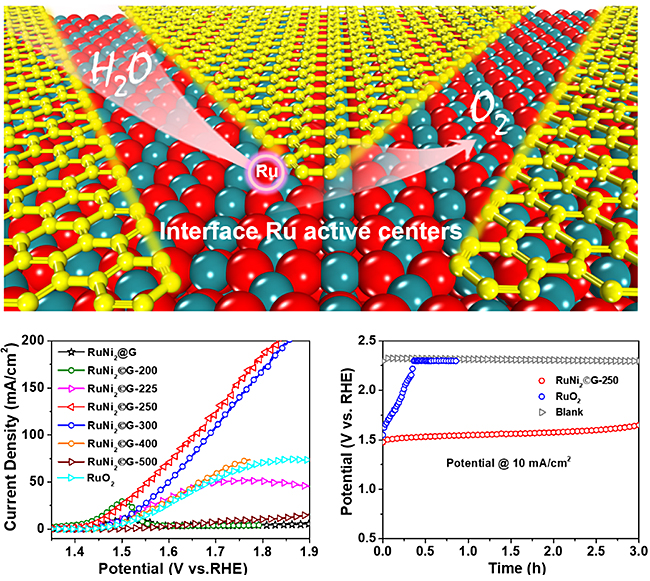
Interface Ru active centers for high-efficiency oxygen evolution reaction. (Image by CUI Xiaoju and GAO Hehua)
The above work was published as a communication in Adv. Mater. It was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund of Chinese Academy of Sciences, Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM), and the National Postdoctoral Program for Innovative Talents. (Text by CUI Xiaoju and GAO Hehua)