Recently, a research team led by Prof. DENG Dehui in the State Key Laboratory of Catalysis of the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and their collaborators explored the reaction mechanisms of electrocatalytic CO2 reduction reaction (CO2RR) to CO using well-defined metal-N4 sites.
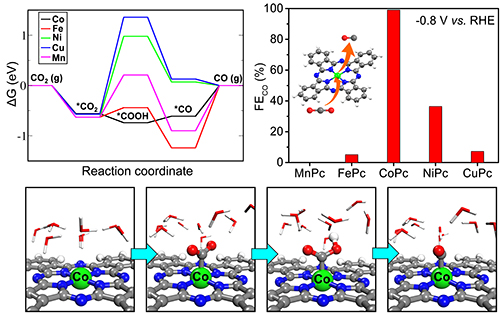
Calculated free energy diagram of the intermediate structures and CO Faradaic efficiency at -0.8 V versus RHE for all MePcs. (Image by ZHANG Zheng and GAO Hehua)
Metal-containing nitrogen-doped carbon (metal-N-C) was found exhibiting promising electrocatalytic activity for CO2RR to CO. However, due to the great challenge in controllable synthesis of uniform and well-defined metal-N-C structures, the active sites and the reaction mechanisms are still under debate and need to be clarified. In addition, the optimum metal-N-C catalyst for CO2RR is still not determined.
Based on previous work on metal-N4 active sites and related catalytic reactions, the scientists found that metal phthalocyanines (MePcs) with well-defined metal-N4 structures could be used as model catalysts to study the underlying active centers and reaction mechanisms of electrochemical CO2RR.
In this study, they combined density functional theory calculations, electrochemical experiments and in situ XAS characterizations to explore the reaction mechanisms of electrochemical CO2 reduction reaction to CO using MePcs with well-defined metal-N4 sites.
Both theoretical and experimental studies identified CoPc as the optimum catalyst for selective CO2RR to CO among all the MePc candidates. The Co site in CoPc served as the active center and its high activity for CO2RR originates from the moderate *CO binding energy on the Co site which accommodated the *COOH formation and the *CO desorption. The optimized CoPc catalyst could achieve a maximal Faradaic efficiency of 99%, and maintained a long-term stability.
This study clearly illustrates the active center, reaction mechanism, and the activity trend on these well-defined metal-N4 sites, and paves a way towards rational design of highly efficient metal-N-C electrocatalysts for CO2RR.
The research entitled “Understanding the reaction mechanisms of well-defined metal-N4sites in electrocatalytic CO2reduction” was published in Angew. Chem. Int. Ed. (DOI: 10.1002/anie.201808593).
This work was conducted in collaboration with Dr. XIAO Jianping from Westlake University, Prof. TIAN Zhongqun and Prof. WANG Ye from Xiamen University, and Prof. SI Rui from Shanghai Institute of Applied Physics of the Chinese Academy of Sciences.
This work was supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text by ZHANG Zheng and GAO Hehua)