Electrochemical CO2 reduction reaction (CO2RR), driven by the sustainable electric energies, can convert the carbon dioxide into valuable chemicals and fuels.
Scientists prepared a hydrocerussite [Pb3(CO3)2(OH)2] as the electrocatalyst of CO2RR, which increased the Faradaic efficiency to 96% for formate production. However, the reaction mechanism and active site of it remain unclear.
Recently, a research group led by Prof. XIAO Jianping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in cooperation with Prof. ZHANG Bin from Tianjin University, proposed a new approach to study the reaction mechanism and active site for electrochemical CO2RR via reaction phase diagram.
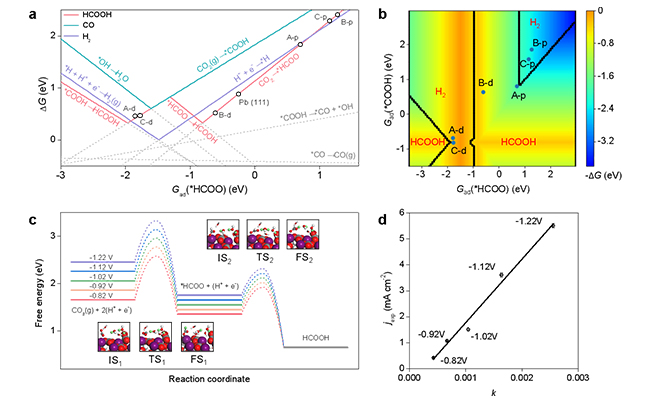
Reaction phase diagram (a, b), free energy diagram (c) and liner correlation between the experimantal current density and theoretical charge transfer rate (d) (Image by LONG Jun)
The scientists conducted density functional theory calculations to understand the reaction mechanism and active site.
According to the bulk structure of hydrocerussite, three terminations were possible to be exposed. For each termination, the vacancy defect of CO3- or OH- might be formed since hydrocerussite was in situ produced. Therefore, six different reaction sites could be obtained.
The scientists found that the adsorption energies of involved intermediates had good correlation on these sites, based on which the reaction phase diagram was constructed with the adsorption energy of *HCOO as the descriptor. They observed two activity volcanos for formate production because the reaction could follow either a *HCOO or *COOH mechanism.
Compared with experimental results, the vacancy site of termination B was determined as the active site, which showed the high activity and selectivity simultaneously.
Besides, the charge transfer rate over this site was calculated by microkinetic simulation, which exhibited a nice linear correlation with the experimental formate partial current density, confirming the above calculations and analysis.
The descriptors-based method can also be generalized to other reaction systems, providing a new way to study the reaction mechanism and the product selectivity of active sites.
This study was published in Nature Communications. It was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of Chinese Academy of Sciences and Liaoning Revitalization Talents program. (Text by LONG Jun)