Precisely regulating the electronic structures of metal active species is highly desirable for electrocatalysis. Carbon-based substrates with inert surface provide weak metal-support interaction and thus are unable to modulate their electronic structures effectively.
Recently, a group led by Prof. LIU Jian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. ZHOU Si from Dalian University of Technology and Prof. LIANG Ji from Tianjin University, used single atoms to modify the carbon substrate to enhance the interaction between substrate and supported metal nanoparticles. The method could precisely tailor the electronic structures of metal nanoparticles.
This study was published in Angew. Chem. Int. Ed. on May 8.
The cobalt single-atom doped carbon-supported Ru showed ultrahigh catalytic activity and stability (Image by SU Panpan)
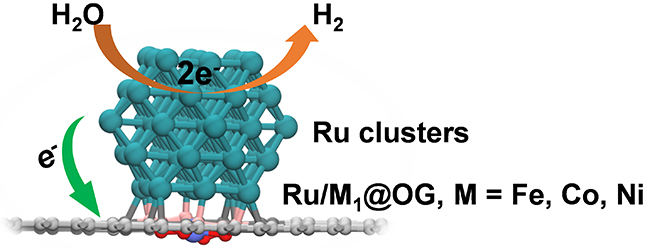
The researchers dispersed single Co atoms on carbon substrate to enhance the interaction between substrate and the ruthenium (Ru) nanoreactor, which enabled remote regulation of the electronic states of Ru nanoparticles, and thereby tuning their electrocatalytic activity.
They took hydrogen evolution reaction (HER) as a model reaction, the cobalt single-atom doped carbon-supported Ru nanoreactor showed ultrahigh catalytic activity and stability.
Theoretical calculations showed that decoration of oxygen-containing graphene by single metal atoms induced electron redistribution on the carbon surface. The carbon atoms surrounding the single atoms were electron deficient, which enhanced the electron transfer from Ru nanoparticles to the carbon substrate, and thereby optimized the binding capability of Ru nanoreactor and its HER performance.
"This work realizes the efficient produce of green hydrogen using non-Pt electrocatalysts and provides a new strategy for steering the catalytic behavior of carbon-supported metal nanocatalysts in a more flexible and nondestructive manner," said by Prof. LIU. (Text by SU Panpan)