Electrocatalytic decomposition of water is significant in clean energy system. The oxygen evolution reaction (OER) is the bottleneck of electrochemical water splitting due to its slow kinetic characteristic.
Electrochemical water splitting is divided as alkaline electrolyzer and acid electrolyzer with harsh environment. Ruthenium oxide is a typical acidic OER catalyst, and its activity is poor in alkaline solution.
Recently, a group led by Prof. WU Zhongshuai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed two-dimensional unsaturated ruthenium oxide/graphene heterostructures with high activity and stability for pH-universal oxygen evolution.
The study was published in Nano Energy on August 1.
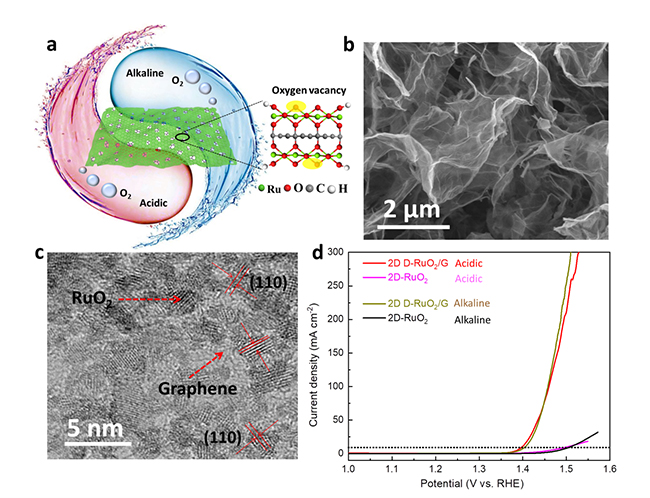
Schematic diagram (a), SEM image (b), HRTEM image (c), OER performance in both acidic and alkaline solutions (d) of 2D D-RuO2/G (Image by LI Yaguang)
The OER activity of ruthenium oxide is closely related to the coordination structure of Ru sites. In general, the Ru sites in perfect ruthenium oxide are RuO6 coordination structure. However, it is reported that the RuO5 structure with oxygen deficiency has better OER activity compared with RuO6.
Therefore, the key of improving OER activity of ruthenium oxide is to create Ru sites with oxygen deficiencies. In addition, the ruthenium oxide electrode has high resistance of 2000 Ω level, resulting in limited OER activity.
The scientists proposed a confined oxygenation strategy using the finite oxygen species from graphene oxide to synthesize two dimensional (2D) heterostructures of intrinsically oxygen defective ruthenium oxide nanocrystals uniformly grown on graphene (2D D-RuO2/G).
Due to the strong coordination between graphene oxide and Ru precursor, the two-dimensional structure had an ultrathin thickness of 9 nm, high specific surface area of 125 m2/g, low resistance of 4 Ω level, and intrinsic RuO5 oxygen defective structure evidenced by synchrotron radiation.
Consequently, 2D D-RuO2/G exhibited an ultralow current density of 10 mA/cm2 at the overpotential of 169 and 175 mV in acidic and alkaline electrolytes respectively, far exceeding the state-of-the-art of pH-universal OER electrocatalysts.
Theoretical studies indicated that intrinsic defective RuO5 sites could enhance the adsorption and accelerate the decomposition of hydroxyl groups to boost the OER activity.
This work provides the new opportunities to develop 2D advanced defective OER electrocatalysts in all-pH electrolytes.
This work was supported by National Natural Science Foundation of China, National Key R&D Program of China. (Text by LI Yaguang and HOU Xiaocheng)