Scientists from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Scientists (CAS), collaborated with Tsinghua University and Arizona State University, revealed a much more pronounced water promotional effect on CO oxidation on Au single-atom sites than on interfacial sites of Au nanocatalyst due to their different local atomic structures and electronic properties. The results were published in Nature Communications.
Scheme illustration of single-atom sites and interfacial sites of nanocatalyst (above); Different water promoting effect on CO oxidation on single-atom catalysts and nanocatalyst (below).
Supported metal catalysts have played a central role in the modern chemical industry. The synergy between a support and supported metal is often critical in determining the performance of catalysts because the interfacial perimeter sites in many cases serve as the dominant active sites.
Heterogeneous single-atom catalysts (SACs), consisting of isolated metal single atoms dispersed onto a support, maximize the number of interfacial perimeter sites and are expected to provide unique opportunities for catalyzing chemical transformations. However, due to the quantum size and ensemble effects of metal clusters and particles, and the differences between SACs and nanocatalysts in electronic and support effects as well as metal support interaction, it is still an open question whether the active sites in SACs possess similar catalytic properties to those of the interfacial sites in supported metal NPs or cluster catalysts.
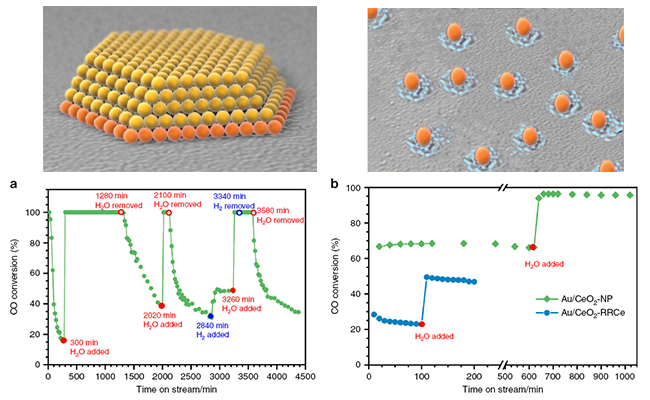
In this work, scientists discovered that CO oxidation on the single-atom sites was dramatically promoted by the presence of water whereas on interfacial sites of nanocatalyst the promoting effect was much weaker.
Computational modeling based on density functional theory (DFT) revealed that the significant difference in the water promoting effect originated from the different active-site structure and electronic properties between Au single atoms in the Au SAC and the interfacial Au atoms in supported Au NP catalysts.
The positively charged, non-zero valent Au atom in the Au1/CeO2 SAC was more variable in oxidation states as an electron acceptor, which offered a more efficient channel for the CO and OH reaction pathway with the presence of OH groups (or water molecules). In contrast, the near zero-valent Au interfacial atoms in supported Au NPs did not seem to favor such oxidation state change, thus significantly hindering the CO and OH reaction pathway.
This work not only offers an avenue to develop highly efficient catalyst for CO oxidation with the presence of water by fabricating suitable single-atom catalysts, but also provides an example to understand the difference between SAC and the interfacial sites in supported metal NPs or cluster catalysts. (Text by CHEN Fang)