Ethylene methoxycarbonylation reaction is the key process in the Alpha-route to produce methyl methacrylate industrially. This approach has the advantages of using widely available raw materials, having high atomic efficiency, as well as high selectivity when compared with traditional methodologies such as acetone-cyanohydrins, isobutene oxidation and ethylene hydroformylation.
However, ethylene methoxycarbonylation reaction still employs homogeneous Pd-P complexes as catalysts, and uses strongly corrosive acids as promoters, which has difficulty in the recovery of the catalysts and raise environmental concerns.
Recently, a research group led by Profs. ZHANG Tao and WANG Aiqin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) realized the ethylene methoxycarbonylation reaction over Pt1/MoS2 single-atom catalyst (SAC).
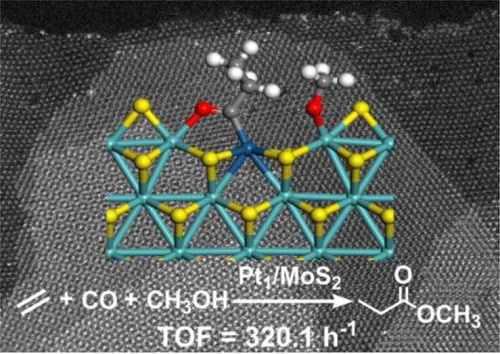
Pocket-like ensemble of Mo-S-Pt1-S-Mo sites for ethylene methoxycarbonylation by M-H mechanism (Image by WANG An)
As a bridge between homogeneous and heterogeneous catalysis, SACs are regarded as new opportunities. In this study, the researchers fabricated MoS2 nanosheets supported Pt SAC, where Pt formed multi-functional Mo-S-Pt-S-Mo entities with Mo atoms on the support.
They found that the metal-support concerted catalysis, Pt1/MoS2 presented good catalytic performances in ethylene methoxycarbonylation reaction under acid promoter-free conditions. The turnover frequency (TOF) reached 320 h-1, comparable to some homogeneous catalysts. Pt1/MoS2 could be reused without significant decay in catalytic activity.
"Our study provides a new way to design and develope efficient heterogeneous catalysts for alkoxycarbonylation reactions of alkenes," said Prof. WANG.
The above work was supported by the National Natural Science Foundation of China, the CAS Project for Young Scientists in Basic Research, and the National Key Projects for Fundamental Research and Development of China.