Recently, a research group led by Prof. WANG Aiqin and Prof. ZHANG Tao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University unravelled the coordination structure-performance relationship in single-atom catalyst (SAC).
By using a new ethanediamine complexation & rapid thermal treatment (RTT) in inert atmosphere method, they finely tuned the coordination environment of single atom by merely adjusting the RTT temperature without changing the single-atom dispersion. Consequently, the relationship among single atom coordination environment, electron structure and catalytic performance was established. This work was published in Nat. Commun..
In 2011, Prof. ZHANG, Prof. LI, and Prof. LIU Jingyue from Arizona State University reported Pt1/FeOX SAC, and on this basis the concept of "single atom catalysis" was proposed. In the past 8 years, SAC has rapidly become the frontier in the field of catalysis.
As a special type of supported metal catalyst, in SAC the active metal species are exclusively dispersed as isolated single atoms via bonding chemically with the surrounding atoms from the support. However, unlike metal organic complexes, the rigid linkage structure in SAC makes it a great challenge to modulate the coordination environment of the single atom center.
The correlation between coordination structure and catalytic performance. (Image by REN Yujing)
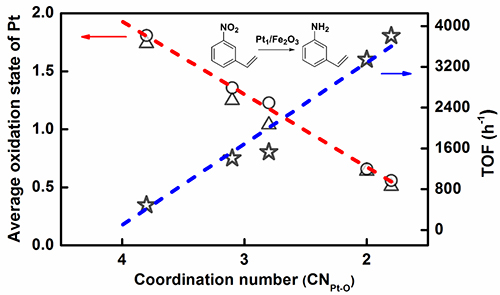
On the basis of previous research work, by innovating new SAC preparation method, Prof. WANG and Prof. ZHANG developed an efficient ethanediamine complexation & rapid thermal treatment (RTT) in inert atmosphere method. Not only the high loading Pt1/Fe2O3-T (T: 500 °C to 600 °C) SAC was successfully prepared, but also the coordination environment of Pt single atom could be finely tuned by merely adjusting the RTT temperature without changing the single-atom dispersion. The higher RTT temperature resulted in the lower coordination number (CN) of Pt-O in the first shell.
By further using the Pt-O CN of single atom Pt center as a descriptor to predict the electron state of Pt center and corresponding catalytic hydrogenation activity, a structure-performance relationship was established. The lower the CN of Pt-O is, the lower the average oxidation state of Pt and the higher the hydrogenation activity are.
This coordination environment tunability of the single atom center makes SAC a bridge between homogeneous catalysis and heterogeneous catalysis, which opens up a new way for designing high active and selective hydrogenation catalysts.
This work was supported by the National Science Foundation of China (NSFC), the Strategic Priority Research Program of the Chinese Academy of Sciences and the National Key Projects for Fundamental Research and Development of China. (Text by REN Yujing)