Recently, Dr. SUN Jian and Dr. YU Jiafeng from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed a new technology to freeze lattice oxygen atoms into metastable state in order to enhance their activity in redox cycle. The CO oxidation reaction rate was accelerated by 10 times compared to the conventional catalysts. Their results were published in Chemical Science.
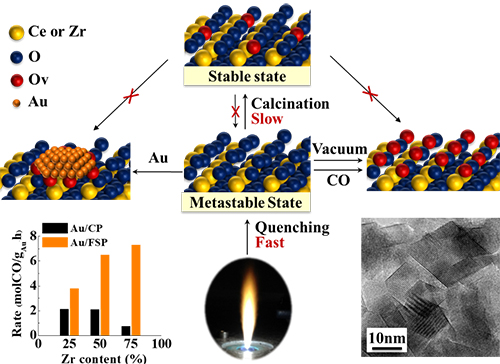
Oxygen release in redox cycle is enhanced due to the formation of disordered lattice oxygen in the quenching process. (Image by YU Jiafeng)
The redox cycle, including lattice oxygen release and oxygen exchange with vacancies, is an important process in catalytic oxidation reactions.
Generally, the rate-determining step of this process is lattice oxygen release. Therefore, the enhancement of lattice oxygen activity to accelerate redox cycle is one of the efficient methods to improve catalytic oxidation reactions.
Researchers disturbed the lattice oxygen atoms of Ce-Zr solid solution via quenching the as-prepared oxides from high temperature in the flame spray pyrolysis method, weakening the metal-oxygen interactions.
The metastable lattice oxygen atoms could stay stable in fresh catalysts, as well as release in relatively mild conditions, such as reduction at room temperature, vacancy treatment and metal deposition.
The results showed that the produced oxygen vacancies on FSP made oxides are 19 times more than that made by coprecipitation.
This strategy can be applied in the design and the fabrication of improved oxygen storage materials. And this new concept is expected to be widely employed in many oxygen activation reactions in heterogenous catalysis. (Text by YU Jiafeng)