Research News
-
 10 12, 2019Scientists Develop New Technology for Liquid Phase Oxidation of O-xyleneA research group led by Prof. GAO Jin and Prof. XU Jie from the Dalian Institute of Chemical Physics (DICP) of the Chinse Academy of Sciences (CAS) developed a new technology for liquid phase oxidation and then esterification of o-xylene to produce dimethyl phthalate.
10 12, 2019Scientists Develop New Technology for Liquid Phase Oxidation of O-xyleneA research group led by Prof. GAO Jin and Prof. XU Jie from the Dalian Institute of Chemical Physics (DICP) of the Chinse Academy of Sciences (CAS) developed a new technology for liquid phase oxidation and then esterification of o-xylene to produce dimethyl phthalate.
A research group led by Prof. GAO Jin and Prof. XU Jie from the Dalian Institute of Chemical Physics (DICP) of the Chinse Academy of Sciences (CAS) developed a new technology for liquid phase oxidation and then esterification of o-xylene to produce dimethyl phthalate. In cooperation with Shaanxi Yanchang Petroleum (Group) Co., Ltd., they built the first 2000 t/a industrial test equipment.
The industrial test was completed in July 2019. And recently, it passed the on-site assessment by experts organized by China Petroleum and Chemical Industry Federation.
The First 2000 t/a Industrial Test Equipment for Liquid Phase Oxidation of o-Xylene (Photo by HUANG Yizheng)
Compared with the traditional gas phase oxidation process, the oxidation reaction temperature in the new technology was reduced by 160-180 °C, and the yield of dimethyl phthalate was increased by 12-17 percentage. It could greatly reduce the consumption of o-xylene and the emission of CO2.
On September 27th, the technology passed the appraisal of scientific and technological achievements organized by China Petroleum and Chemical Industry Federation in Beijing. Experts of the appraisal committee agreed that the technology had independent intellectual property rights, advanced technology, strong innovation, and the comprehensive technical level had reached international leading level.
This work was supported by Major Project of National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, etc. (Text by HUANG Yizheng and FENG Tianshi) -
 10 12, 2019Scientists Unravel Coordination Structure-performance Relationship in Single-atom CatalystRecently, a research group led by Prof. WANG Aiqin and Prof. ZHANG Tao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University unravel the coordination structure-performance relationship in single-atom catalyst (SAC).
10 12, 2019Scientists Unravel Coordination Structure-performance Relationship in Single-atom CatalystRecently, a research group led by Prof. WANG Aiqin and Prof. ZHANG Tao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University unravel the coordination structure-performance relationship in single-atom catalyst (SAC).
Recently, a research group led by Prof. WANG Aiqin and Prof. ZHANG Tao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University unravelled the coordination structure-performance relationship in single-atom catalyst (SAC).
By using a new ethanediamine complexation & rapid thermal treatment (RTT) in inert atmosphere method, they finely tuned the coordination environment of single atom by merely adjusting the RTT temperature without changing the single-atom dispersion. Consequently, the relationship among single atom coordination environment, electron structure and catalytic performance was established. This work was published in Nat. Commun..
In 2011, Prof. ZHANG, Prof. LI, and Prof. LIU Jingyue from Arizona State University reported Pt1/FeOX SAC, and on this basis the concept of "single atom catalysis" was proposed. In the past 8 years, SAC has rapidly become the frontier in the field of catalysis.
As a special type of supported metal catalyst, in SAC the active metal species are exclusively dispersed as isolated single atoms via bonding chemically with the surrounding atoms from the support. However, unlike metal organic complexes, the rigid linkage structure in SAC makes it a great challenge to modulate the coordination environment of the single atom center.
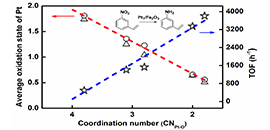
The correlation between coordination structure and catalytic performance. (Image by REN Yujing)
On the basis of previous research work, by innovating new SAC preparation method, Prof. WANG and Prof. ZHANG developed an efficient ethanediamine complexation & rapid thermal treatment (RTT) in inert atmosphere method. Not only the high loading Pt1/Fe2O3-T (T: 500 °C to 600 °C) SAC was successfully prepared, but also the coordination environment of Pt single atom could be finely tuned by merely adjusting the RTT temperature without changing the single-atom dispersion. The higher RTT temperature resulted in the lower coordination number (CN) of Pt-O in the first shell.
By further using the Pt-O CN of single atom Pt center as a descriptor to predict the electron state of Pt center and corresponding catalytic hydrogenation activity, a structure-performance relationship was established. The lower the CN of Pt-O is, the lower the average oxidation state of Pt and the higher the hydrogenation activity are.
This coordination environment tunability of the single atom center makes SAC a bridge between homogeneous catalysis and heterogeneous catalysis, which opens up a new way for designing high active and selective hydrogenation catalysts.
This work was supported by the National Science Foundation of China (NSFC), the Strategic Priority Research Program of the Chinese Academy of Sciences and the National Key Projects for Fundamental Research and Development of China. (Text by REN Yujing) -
 10 11, 2019Scientists Observe a Phonon Bottleneck for Hot Electron Cooling in Doped Quantum DotsA research group led by Prof. WU Kaifeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) recently reported the first observation of a phonon bottleneck for hot electron relaxation copper-doped colloidal quantum dots.
10 11, 2019Scientists Observe a Phonon Bottleneck for Hot Electron Cooling in Doped Quantum DotsA research group led by Prof. WU Kaifeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) recently reported the first observation of a phonon bottleneck for hot electron relaxation copper-doped colloidal quantum dots.
A research group led by Prof. WU Kaifeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) recently reported the first observation of a phonon bottleneck for hot electron relaxation copper-doped colloidal quantum dots.
In conventional solar cells, the excessive energy of carriers above the band gap of a semiconductor is lost to heat due to rapid cooling (thermalization) of hot carriers via emission of phonons. This is a major reason for the well-known Shockley–Queisser limit for solar cell efficiency.
Hot carrier extraction to selective contacts has been envisioned as a promising means to overcome this limit, which has the potential to boost power conversion efficiency of solar cells to as high as 66%. The challenge in efficient hot carrier extraction lies in that intraband cooling of hot carriers usually occurs on a sub-ps timescale.
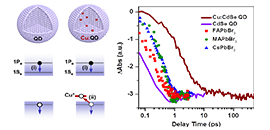
Femtosecond hole capturing by copper dopants in colloidal cadmium selenide quantum dots slows down their hot electron cooling by more than 30-fold. (Image by WANG Lifeng)
It is thus essential to suppress the rate of hot carrier cooling. Semiconductor quantum dots (QDs) were predicted to exhibit a “phonon bottleneck” for hot electron relaxation as their quantum-confined electrons would couple very inefficiently to phonons. However, typical CdSe QDs, for example, still exhibit sub-picosecond hot electron cooling, by passing the phonon bottleneck possibly via an Auger-like process whereby the excessive energy of the hot electron is transferred to the hole.
Realizing the detrimental role of the Auger-type cooling mechanism, the key to enabling a phonon bottleneck and to prolonging hot electron lifetime is to decouple the electron from the hole and thus to suppress energy transfer between them.
In this work, WU and his coworkers showed that, in copper-doped CdSe QDs prepared by a simple one-pot colloidal synthesis, photogenerated holes were captured by copper dopants on a femtosecond timescale (<< 390 fs). This femtosecond hole capturing thus competed favorably with the sub-ps Auger-type, electron-to-hole energy transfer. Hole capturing also likely led to electron wavefunction shrinkage, suppressing hot electron relaxation via nonadiabatic transitions induced by surface ligands.
As a result, they observed a lifetime of ~8.6 ps for 1Pe hot electrons which is more than 30-fold longer than that in same-sized, undoped CdSe QDs (~0.25 ps). Moreover, the hot electron energy loss rate of copper-doped QDs (0.075 eV/ps) was found to be more than 20-fold slower than those of various types of lead halide perovskites being intensively studied of late for hot carrier devices.
The observation of a phonon bottleneck in QDs not only is a verification of a long-existing theoretic prediction, but also offers an exciting opportunity of utilizing the long-lived hot electrons for high-efficiency photovoltaics and photocatalysis.
The above results were recently published in Nat. Commun..
This work was supported by the Ministry of Science and Technology of China, the Chinese Academy of Sciences, the National Natural Science Foundation of China and the LiaoNing Revitalization Talents Program. (Text by WANG Lifeng) -
 10 09, 2019Highly Efficient Triplet Acceptor Developed for Polarity Tuned Triplet-triplet Annihilation UpconversionA research group led by Prof. HAN keli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a highly efficient triplet acceptor for polarity tuned triplet-triplet annihilation upconversion.
10 09, 2019Highly Efficient Triplet Acceptor Developed for Polarity Tuned Triplet-triplet Annihilation UpconversionA research group led by Prof. HAN keli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a highly efficient triplet acceptor for polarity tuned triplet-triplet annihilation upconversion.
A research group led by Prof. HAN keli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a highly efficient triplet acceptor for polarity tuned triplet-triplet annihilation upconversion (TTA UC). The study was published as a letter in The Journal of Physical Chemistry Letters.
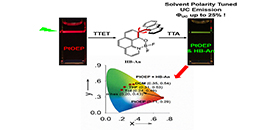
Triplet–triplet annihilation upconversion (TTA UC) was intensively investigated for developing efficient photosensitizers and emitters. (Image by LIU Ya)
Photon upconversion by triplet-triplet annihilation with advantages of low excitation power and high quantum yields has applications in organic light emitting diode, photo(electro)chemistry, solar cell devices and biological imaging.
Recently, the developing of new families of upconversion emitters/triplet energy acceptors for TTA UC has attracted great attention. However, the excited state energy levels of the emitters could only be tuned by chemical modifications. Emission wavelength tunable TTA UC system with only one emitter has not been reported yet.
In this study, the scientists constructed an emission wavelength tunable TTA UC system using a novel hetero-bichromophore dyad with solvatochromic emission as triplet acceptor/emitter. High TTA UC quantum yield (Φuc) up to 25.5% was achieved and the upconversion emission of the solvent polarity tuned TTA UC system could be fine-tuned from cyan to yellow by changing the media polarity.
In addition, they investigated the detail photophysical properties of the HB-An dyad by using steady-state and femtosecond/nanosecond transient absorption spectroscopies and quantum chemical calculations, in order to uncover the relationship between molecular structure and its excited state properties.
The study constructs efficient emission wavelength tunable TTA UC systems and is beneficial for developing new family of TTA UC emitters.
The above work was supported by the National Natural Science Foundation of China. And it is dedicated to the 70th anniversary of DICP. (Text by Liu Ya) -
 10 08, 2019Scientists Review Progress of Confinement Catalysis with 2D Materials for Energy ConversionA research team led by Prof. DEND Dehui and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences reviewed the progress of confinement catalysis with 2D materials for energy conversion.
10 08, 2019Scientists Review Progress of Confinement Catalysis with 2D Materials for Energy ConversionA research team led by Prof. DEND Dehui and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences reviewed the progress of confinement catalysis with 2D materials for energy conversion.
A research team led by Prof. DEND Dehui and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences reviewed the progress of confinement catalysis with 2D materials for energy conversion. The article was published in Advanced Materials.
Confinement Catalysis with 2D Materials for Energy Conversion. (Image by TANG Lei and GAO Hehua)
The unique electronic and structural properties of 2D materials have triggered wide research interest in catalysis. The lattice of 2D materials and the interface between 2D covers and other substrates provide intriguing confinement environments for active sites, which has stimulated a rising area of “confinement catalysis with 2D materials.”
Fundamental understanding of confinement catalysis with 2D materials can favor the rational design of high-performance 2D nanocatalysts. Confinement catalysis with 2D materials has found extensive applications in energy-related reaction processes, especially in the conversion of small energy-related molecules such as H2, O2, CH4, CO, CO2, H2O, and CH3OH.
Two representative strategies, i.e., 2D lattice-confined single atoms and 2D cover-confined metals, have been applied to construct 2D confinement catalytic systems with superior catalytic activity and stability.
Scientists summarized recent advances in the design, applications, and structure–performance analysis of two 2D confinement catalytic systems. The different routes for tuning the electronic states of 2D confinement catalysts were highlighted and perspectives on confinement catalysis with 2D materials toward energy conversion and utilization in the future were provided.
The above work was supported by Ministry of Science and Technology of China, Major Project of National Natural Science Foundation of China, Key Research Program of Frontier Sciences of CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM), etc. It was dedicated to the 70th anniversary of DICP. (Text by TANG Lei and GAO Hehua) -
 10 08, 2019Scientists Reveal Dynamic Mechanism of All-Inorganic Low-Dimensional Cesium Copper Halide NanocrystalsScientists revealed the dynamic mechanism of all-Inorganic low-dimensional cesium copper halide nanocrystals.
10 08, 2019Scientists Reveal Dynamic Mechanism of All-Inorganic Low-Dimensional Cesium Copper Halide NanocrystalsScientists revealed the dynamic mechanism of all-Inorganic low-dimensional cesium copper halide nanocrystals.
A research group led by Prof. HAN Keli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the dynamic mechanism of all-Inorganic low-dimensional cesium copper halide nanocrystals. This work was published in Angew. Chem. Int. Ed..
Organic-inorganic metal halide semiconductors have shown promising prospect in a variety of optoelectronic applications due to their excellent optical and electronic properties. The exceptional structural tunability of such materials enables them to form various types of crystal structures, from three-dimensional (3D) networks to two-dimensional (2D) layers, one-dimensional (1D) chains and ultimately zero-dimensional (0D) isolated structures at the molecular level.
Although significant progress has been realized in the study of 3D and 2D metal halides, 1D and 0D metal halides, which exhibit unique photophysical properties due to the strong quantum confinement effects, have not been fully investigated to date.
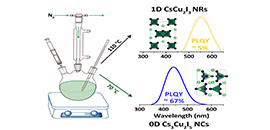
Colloidal synthesis of cesium copper halide nanocrystals.
The scientists reported the colloidal synthesis of all-inorganic low-dimensional cesium copper halide nanocrystals (NCs) by adopting a hot-injection approach. Using the same reactants and ligands, but at different reaction temperatures, both 1D CsCu2I3 nanorods (NRs) and 0D Cs3Cu2I5 NCs could be prepared.
The as-prepared 1D CsCu2I3 NRs showed a weak yellow emission with a PLQY of 5%, while 0D Cs3Cu2I5 NCs exhibited a bright blue emission with a PLQY up to 67%. From 1D CsCu2I3 to 0D Cs3Cu2I5 , the reduced dimensionality made the excitons more localized as indicated by density functional theory, which accounted for the strong emission of 0D Cs3Cu2I5 NCs.
Subsequent optical characterization revealed that the highly luminescent, strongly Stokes-shifted broadband emission of 0D Cs3Cu2I5 NCs arose from the self-trapped excitons (STEs).
Their findings not only present a method to control the synthesis of low-dimensional cesium copper halide nanocrystals but also highlight the potential of 0D Cs3Cu2I5 NCs in optoelectronics.
This work was supported by the key research project of National Natural Science Foundation. It was dedicated to the 70th anniversary of DICP. (Text and Image by Cheng Pengfei)