Scientists from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and Peking University presented a cryo-EM structure of a tetrameric Photosystem I complex and revealed the mechanism of tetrameric assembly of Photosystem I and its physiological functions. Their findings were published in Nature Plants.
Photosynthesis is the main way to convert solar energy into chemical energy in nature. There are two large protein complexes, Photosystem I (PSI) and Photosystem II (PSII), on thylakoid membranes of higher plants and eukaryotic algae to accomplish a series of photosynthetic steps, such as photoinduced electron transport and ATP biosynthesis. PSI and PSII of different species are highly conserved and perform physiological functions in different oligomeric states on thylakoid membranes. Previous studies mainly focused on the trimeric PSI.
Prof. GAO Ning’s group from Peking University provided a high-resolution cryo-EM structure of the tetrameric PSI complex found in heterocyst-forming cyanobacterial species.
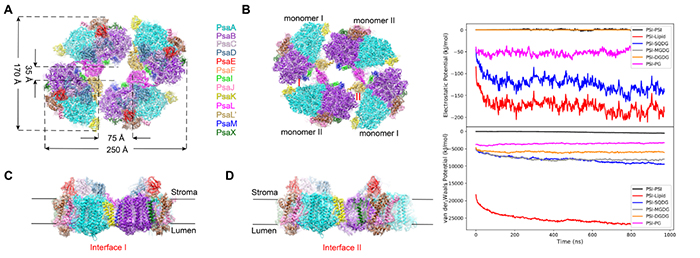
3-D structure of tetrameric PSI complex and time-course of the nonbonded interactions during the assembly. (Image by ZHONG Qinglu)
Prof. LI Guohui’s group from DICP performed molecular dynamic simulations to explore the self-assembled process of tetrameric PSI on thylakoid membranes and revealed its assembled mechanism.
Four PSI monomers organized in a dimer of dimer, formed two distinct interfaces that were largely mediated by specifically orientated polar lipids, such as sulfoquinovosyl diacylglycerol. The structure depicted a more closely connected network of chlorophylls across monomer interfaces than those seen in trimeric PSI from thermophilic cyanobacteria, possibly allowing a more efficient energy transfer between monomers.
Prof. ZHAO Jindong’s group from Peking University revealed a functional link of photosystem I oligomerization to cyclic electron flow and thylakoid membrane organization from physiological data.
This research was supported by the National Natural Science Foundation of China. (Text by ZHONG Qinglu)