Research News
-
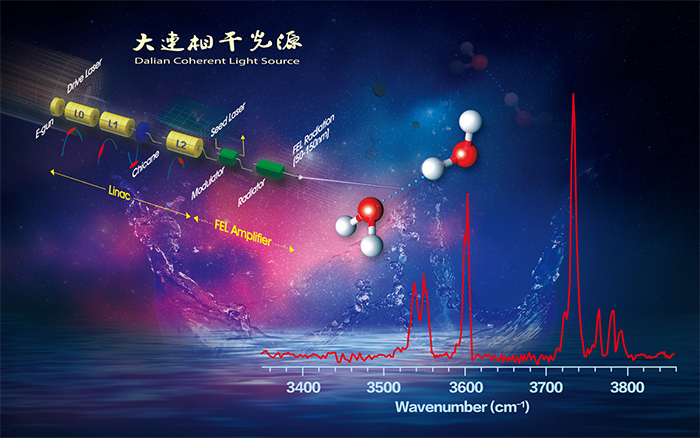 02 19, 2020Scientists Develop Infrared Spectroscopy of Neutral Water Dimer Based on a Tunable Vacuum Ultraviolet Free Electron LaserScientists developed infrared spectroscopy of neutral water dimer based on a tunable vacuum ultraviolet free electron laser. Their studies help to resolve the controversy of the exact vibrational assignment of each band feature of water dimer. The results were published in the journal The Journal of Physical Chemistry Letters.Researchers led by Prof. JIANG Ling, Prof. YANG Xueming and Prof. ZHANG Donghui from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, collaborated with Prof. LI Jun from Tsinghua University have developed infrared spectroscopy of neutral water dimer based on a tunable vacuum ultraviolet free electron laser. Their studies help to resolve the controversy of the exact vibrational assignment of each band feature of water dimer. The results were published in the journal The Journal of Physical Chemistry Letters. Clusters consisting of a few to hundreds of atoms exhibit interesting size-dependent properties and are the bridge between molecules and condensed phase bulks. Optical spectroscopy of gas-phase clusters provides detailed structural and dynamical information that is difficult to extract from bulk measurements. Over the past several decades, enormous efforts were devoted to the spectroscopic study of charged clusters, which allow easy size-selection and detection. In contrast, neutral clusters have presented major experimental challenges, because the absence of a charge makes it difficult for size-selection and detection. Recently, Dalian Coherent Light Source (DCLS) facility developed by DICP, which delivers VUV Free Electron Laser (FEL) with a continuously tunable wavelength region between 50 and 150 nm and high pulse energy. Inasmuch as clusters with different sizes have different ionization energies, the tunable VUV-FEL light paves the way for selectively ionizing a given neutral cluster free of confinement, thus facilitating realization of their size selectivity. This unique VUV-FEL facility makes it possible to study the IR spectroscopy of confinement-free, neutral clusters via the IR-VUV scheme. Researchers led by Prof. JIANG Ling and Prof. YANG Xueming from DICP have built an IR-VUV spectroscopy apparatus based on the VUV-FEL and have successfully measured the IR spectra of the water dimer. The water cluster is considered here because of its central role in scientific disciplines ranging from geology to astronomy to biology. The water dimer is a prototype system for the study of the cooperative hydrogen bond (HB) in liquid water, which has proven to be difficult to adequately capture through bulk experiments or theory. Quantum mechanical calculations on a 12-dimensional ab initio potential energy surface have been utilized to simulate the anharmonic vibrational spectra of the water dimer. The anharmonic vibrational calculations reproduced the well-known four bands of water dimer, which correspond to antisymmetric OH stretch of the proton acceptor, free OH stretch of the proton donor, symmetric OH stretch of the proton acceptor, and hydrogen-bonded OH stretch of the proton donor. As shown by the bond distances, bond orders, and hybrid orbitals, the O-H bonds in HHOa is stronger than those in the H-ObH due to electron donation from Oa lone pair to the s(OH)* anti-bonding orbitals, the symmetric and antisymmetric OH vibrational frequencies of HHOa are higher than those of H-ObH. The electronic structure analyses are helpful for understanding the spectroscopic features of OH vibrational modes for different hydrogen bond orientation in more complicated water clusters. As many clusters have their ionization energies in a range accessible by VUV-FEL light source and near threshold ionization can be readily achieved, the VUV-FEL based IR spectroscopy opens a new paradigm for the study of vibrational spectra of a wide variety of neutral clusters. The availability of these new experimental data on the neutral clusters is expected to stimulate further calculations and developments of theoretical methods leading to an improved understanding of the structures and dynamics of these systems.
02 19, 2020Scientists Develop Infrared Spectroscopy of Neutral Water Dimer Based on a Tunable Vacuum Ultraviolet Free Electron LaserScientists developed infrared spectroscopy of neutral water dimer based on a tunable vacuum ultraviolet free electron laser. Their studies help to resolve the controversy of the exact vibrational assignment of each band feature of water dimer. The results were published in the journal The Journal of Physical Chemistry Letters.Researchers led by Prof. JIANG Ling, Prof. YANG Xueming and Prof. ZHANG Donghui from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, collaborated with Prof. LI Jun from Tsinghua University have developed infrared spectroscopy of neutral water dimer based on a tunable vacuum ultraviolet free electron laser. Their studies help to resolve the controversy of the exact vibrational assignment of each band feature of water dimer. The results were published in the journal The Journal of Physical Chemistry Letters. Clusters consisting of a few to hundreds of atoms exhibit interesting size-dependent properties and are the bridge between molecules and condensed phase bulks. Optical spectroscopy of gas-phase clusters provides detailed structural and dynamical information that is difficult to extract from bulk measurements. Over the past several decades, enormous efforts were devoted to the spectroscopic study of charged clusters, which allow easy size-selection and detection. In contrast, neutral clusters have presented major experimental challenges, because the absence of a charge makes it difficult for size-selection and detection. Recently, Dalian Coherent Light Source (DCLS) facility developed by DICP, which delivers VUV Free Electron Laser (FEL) with a continuously tunable wavelength region between 50 and 150 nm and high pulse energy. Inasmuch as clusters with different sizes have different ionization energies, the tunable VUV-FEL light paves the way for selectively ionizing a given neutral cluster free of confinement, thus facilitating realization of their size selectivity. This unique VUV-FEL facility makes it possible to study the IR spectroscopy of confinement-free, neutral clusters via the IR-VUV scheme. Researchers led by Prof. JIANG Ling and Prof. YANG Xueming from DICP have built an IR-VUV spectroscopy apparatus based on the VUV-FEL and have successfully measured the IR spectra of the water dimer. The water cluster is considered here because of its central role in scientific disciplines ranging from geology to astronomy to biology. The water dimer is a prototype system for the study of the cooperative hydrogen bond (HB) in liquid water, which has proven to be difficult to adequately capture through bulk experiments or theory. Quantum mechanical calculations on a 12-dimensional ab initio potential energy surface have been utilized to simulate the anharmonic vibrational spectra of the water dimer. The anharmonic vibrational calculations reproduced the well-known four bands of water dimer, which correspond to antisymmetric OH stretch of the proton acceptor, free OH stretch of the proton donor, symmetric OH stretch of the proton acceptor, and hydrogen-bonded OH stretch of the proton donor. As shown by the bond distances, bond orders, and hybrid orbitals, the O-H bonds in HHOa is stronger than those in the H-ObH due to electron donation from Oa lone pair to the s(OH)* anti-bonding orbitals, the symmetric and antisymmetric OH vibrational frequencies of HHOa are higher than those of H-ObH. The electronic structure analyses are helpful for understanding the spectroscopic features of OH vibrational modes for different hydrogen bond orientation in more complicated water clusters. As many clusters have their ionization energies in a range accessible by VUV-FEL light source and near threshold ionization can be readily achieved, the VUV-FEL based IR spectroscopy opens a new paradigm for the study of vibrational spectra of a wide variety of neutral clusters. The availability of these new experimental data on the neutral clusters is expected to stimulate further calculations and developments of theoretical methods leading to an improved understanding of the structures and dynamics of these systems.
Infrared spectroscopy of neutral water dimer based on a tunable vacuum ultraviolet free electron laser. (Image by JIANG Ling) This work was supported by the National Natural Science Foundation of China (Grant Nos. 21688102, 21673231, and 91645203), the Strategic Priority Research Program of Chinese Academy of Sciences (CAS) (Grant No. XDB17000000), Dalian Institute of Chemical Physics (Grant No. DICP DCLS201702). (Text by JIANG Ling ) -
 02 19, 2020Scientists develop a new gold nanocatalyst with high catalytic activity and excellent stabilityScientists made an important progress in gold nanocatalysis.: They developed an anti-sintering gold nanocatalyst with high catalytic activity. The results were published in Nature Communications.A joint research team led by Prof. HUANG Jiahui Huang and Prof. QIAO Botao Qiao from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), and Prof. SUN Keju Sun from Yanshan University made an important progress in gold nanocatalysis.: They developed an anti-sintering gold nanocatalyst with high catalytic activity. The results were published in Nature Communications. Gold nanocatalysts have exhibited unexpected catalytic activities in many catalytic reactions such as CO oxidation, propylene epoxidation and selective oxidation of alcohols/aldehydes, and thus been be regarded as one kind of promising catalysts for industrial application. However, the commercialization process of gold nanocatalysts is very slow. A major barrier is their low stability stemmed from the easy sintering of gold nanoparticles. Therefore, the development of gold nanocatalysts with excellent stability becomes one of the biggest challenges in nanogold catalysis field. Many efforts have been focused on addressing the sintering issue of gold nanocatalysts and signi?cant progresses have been achieved. Strategies such as using the strong interaction between the metal and support, coating the catalysts by inert oxide, utilizing meso-porous materials to con?ne noble metal particles can effectively improve the sintering resistance of gold nanocatalysts. However, these progresses were achieved at the cost of losing the activity to different extent.
02 19, 2020Scientists develop a new gold nanocatalyst with high catalytic activity and excellent stabilityScientists made an important progress in gold nanocatalysis.: They developed an anti-sintering gold nanocatalyst with high catalytic activity. The results were published in Nature Communications.A joint research team led by Prof. HUANG Jiahui Huang and Prof. QIAO Botao Qiao from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), and Prof. SUN Keju Sun from Yanshan University made an important progress in gold nanocatalysis.: They developed an anti-sintering gold nanocatalyst with high catalytic activity. The results were published in Nature Communications. Gold nanocatalysts have exhibited unexpected catalytic activities in many catalytic reactions such as CO oxidation, propylene epoxidation and selective oxidation of alcohols/aldehydes, and thus been be regarded as one kind of promising catalysts for industrial application. However, the commercialization process of gold nanocatalysts is very slow. A major barrier is their low stability stemmed from the easy sintering of gold nanoparticles. Therefore, the development of gold nanocatalysts with excellent stability becomes one of the biggest challenges in nanogold catalysis field. Many efforts have been focused on addressing the sintering issue of gold nanocatalysts and signi?cant progresses have been achieved. Strategies such as using the strong interaction between the metal and support, coating the catalysts by inert oxide, utilizing meso-porous materials to con?ne noble metal particles can effectively improve the sintering resistance of gold nanocatalysts. However, these progresses were achieved at the cost of losing the activity to different extent.
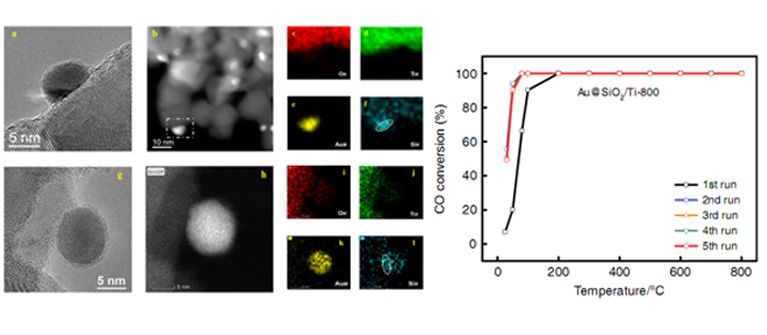
STEM images and EDS mapping of SiO2 modified gold nanocatalyst (Left) and CO conversion versus temperature for different cycles (Right). (Image by ZHANG Junying) Recently, the joint research team prepared a SiO2 modified gold nanocatalyst , by co-deposition of gold and silica precursors on the TiO2 support and subsequent high temperature calcination. This method realizes the mixing of gold species and silica species in atomic level. Through the subsequent calcination process, a SiO2 film with only a thickness of a few atom layers was formed, which was found to cover the surface of gold nanoparticles. This catalyst exhibited highly sintering resistant property and gold nanoparticles could maintain at about 6 nm even after 800 °C calcination. This catalyst also displayed excellent catalytic property and could realize 100% conversion of CO at 0 °C in CO oxidation. Experiments together with computational studies revealed that the SiO2 layer over gold nanoparticles not only prevented the growth of gold nanoparticles, but also promoted the adsorption and activation of O2 during CO oxidation, resulting in a high catalytic activity. The ?nding paves a way for the design and development of gold nanocatalysts with excellent stability and high catalytic activity. This work is supported by National Natural Science Foundation of China and “Transformational Technologies for Clean Energy and Demonstration”, Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by ZHANG Junying) -
 02 16, 2020Scientists Reviewed on Catalytic Lignin Depolymerization to Aromatic ChemicalsScientists recently summarize their researches about lignin catalytic conversion. The article was published and on the Accounts of Chemical Research.Research group led by Prof. WANG Feng from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences was recently invited to summarize their researches about lignin catalytic conversion. The article was published and on the Accounts of Chemical Research.
02 16, 2020Scientists Reviewed on Catalytic Lignin Depolymerization to Aromatic ChemicalsScientists recently summarize their researches about lignin catalytic conversion. The article was published and on the Accounts of Chemical Research.Research group led by Prof. WANG Feng from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences was recently invited to summarize their researches about lignin catalytic conversion. The article was published and on the Accounts of Chemical Research.
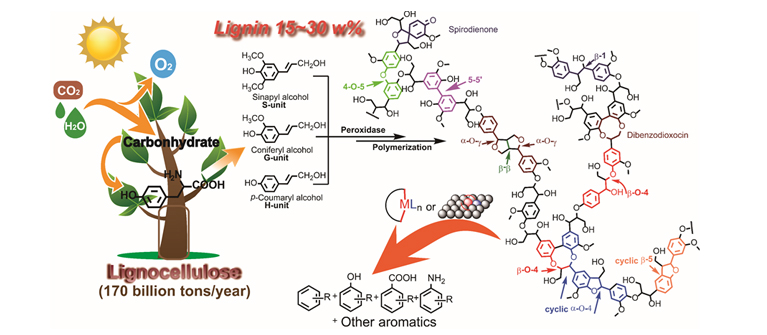
Catalytic Lignin Depolymerization to Aromatic Chemicals. (Image by ZHANG Chaofeng) In recent decades, research on lignin depolymerization and its downstream product transformation has drawn an enormous amount of attention from academia to industry worldwide, aiming at harvesting aromatic compounds from this abundant and renewable biomass resource. Although the lignin conversion can be traced back to the 1930s and various noncatalytic and catalytic methods have been explored to depolymerize lignin via direct lignin conversion research or lignin models conversion studies, the complexity of the lignin structure, various linkages, the high stability of lignin bonds, and the diverse fragments condensation process make lignin depolymerization to monomers a highly challenging task. For the potential practical utilization of lignin, compared with lignin conversion to liquid fuel with extra H2 consumption, maintaining the aromatic structure and preparing high-value aromatic chemicals from renewable lignin is more profitable. Therefore, lignin depolymerization to easy-to-handle aromatic monomers with acceptable conversion and selectivity is of great importance. Prof. WANG and co-workers present their ten years studies on lignin’s catalytic conversion to aromatic chemicals in this article. First, they introduce the research on protolignin depolymerization via a fragmentation–hydrogenolysis process in alcohol solvents. Then, focusing on the catalytic cleavage of lignin C–C and C–O bonds, they shed light on a recapitulative adjacent functional group modification (AFGM) strategy for the conversion of lignin models. AFGM strategy begins with the adjacent functional group modification of the target C–C or C–O bond to directly decrease the bond dissociation enthalpy (BDE) of targeted bonds or generate new substrate sites to introduce the cleavage reagent for further conversion. Subsequently, on the basis of these two concepts from AFGM, they summarize their strategies on lignin depolymerization, which highlight the effects of lignin structure, catalyst character, and reaction conditions on the efficiency of strategies. In short, the key point for lignin depolymerization to aromatics is promoting the lignin conversion and restraining the condensation. Compared with the complex research on direct lignin conversion, this bottom-up research approach, beginning with lignin model research, can make the conversion mechanism study clear and provide potential methods for the protolignin/technical lignin conversion. In addition, one of their perspectives for lignin utilization is that the products from lignin conversion can be used as monomers for artificial polymerization, such as the simple phenol (PhOH) and other potential acid compounds, or that lignin derivative molecules can be used to synthesize high-value synthetic building blocks. This work was supported by the Strategic Priority Research Program of the CAS, National Natural Science Foundation of China, China Scholarship Council and DICP. (Text ZHANG Chaofeng) -
 02 12, 2020Demonstration of novel-concept Pd composite membranes and its applications in NH3 decomposition membrane reactorScientists demonstrated novel Pd composite membranes with a finger-like and gap structure and its application in NH3 decomposition membrane reactor. Their studies were published in the journal Chem. Eng. J.Researchers led by Prof. LI Hui, Prof. CHEN Ping and Asso. Prof. LIU Lin from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences has successfully demonstrated novel Pd composite membranes with a finger-like and gap structure and its application in NH3 decomposition membrane reactor. Their studies were published in the journal Chem. Eng. J. Due to unique permeability to hydrogen and its isotopes, Pd-based membranes play an important role in ultrapure hydrogen generation in semiconductor industry as well as pure hydrogen production for fuel cell application in both vehicular and stationary scenarios. The thermal/chemical stability remains the most critical challenge towards the commercial application of Pd-based composite membranes. Under fuel cell application conditions, the fast response requirements during startup/shutdown process further imposes a high demand on the stability of Pd composite membranes. Thermal stresses may exist between Pd layer and membrane module as well as between Pd layer and porous substrate underneath, which need to be taken into account.
02 12, 2020Demonstration of novel-concept Pd composite membranes and its applications in NH3 decomposition membrane reactorScientists demonstrated novel Pd composite membranes with a finger-like and gap structure and its application in NH3 decomposition membrane reactor. Their studies were published in the journal Chem. Eng. J.Researchers led by Prof. LI Hui, Prof. CHEN Ping and Asso. Prof. LIU Lin from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences has successfully demonstrated novel Pd composite membranes with a finger-like and gap structure and its application in NH3 decomposition membrane reactor. Their studies were published in the journal Chem. Eng. J. Due to unique permeability to hydrogen and its isotopes, Pd-based membranes play an important role in ultrapure hydrogen generation in semiconductor industry as well as pure hydrogen production for fuel cell application in both vehicular and stationary scenarios. The thermal/chemical stability remains the most critical challenge towards the commercial application of Pd-based composite membranes. Under fuel cell application conditions, the fast response requirements during startup/shutdown process further imposes a high demand on the stability of Pd composite membranes. Thermal stresses may exist between Pd layer and membrane module as well as between Pd layer and porous substrate underneath, which need to be taken into account.
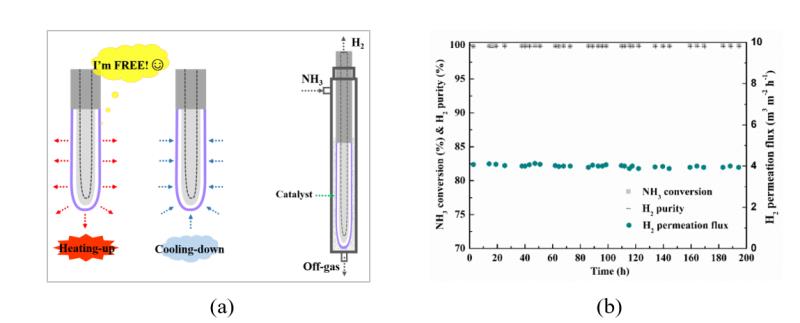
(a) The schematic of novel-configuration Pd composite membrane and NH3 decomposition membrane reactor, (b) A long-term stability test of 200 h of Ru-catalyzed NH3 decomposition Pd membrane reactor at 673 K. (Image by LI Hui, LIU Lin) This study presents a facile and effective approach to develop high performance stainless-steel supported Pd composite membranes with a finger-like and gap structure by coating the finger-like porous stainless-steel support (PSS) with MnCO3 particles, which forms a small gap of ca. 1 μm during subsequent thermal treatment. The stability of this novel configuration was demonstrated under 20–50 fast heating up/cooling down cycles at a maximum ramp rate of 10 K/min, under near practical fuel cell application conditions. Such a structure imitates semi-free-standing bulk Pd membranes, which not only avoids the direct contact between Pd layer and PSS and ensures a high H2 permeance, but also avoids the shear stresses between the metal membrane and module.
The membrane with a finger-like and gap structure was applied in NH3 decomposition membrane reactor, which achieved nearly complete NH3 conversion at a relatively low temperature of 673 K and remained stable for 200 h, exhibiting potential in future applications. This work was supported by the 100-Talent Project of CAS, National Natural Science Foundation of China (Grant No. 21676265; 51501177; 21306183), The Ministry of Science and Technology (MOST) of the People's Republic of China (Grant No. 2016YFE0118300), and the K. C. Wong Education Foundation (GJTD- 2018-06). (Text by LI Hui, LIU Lin) -
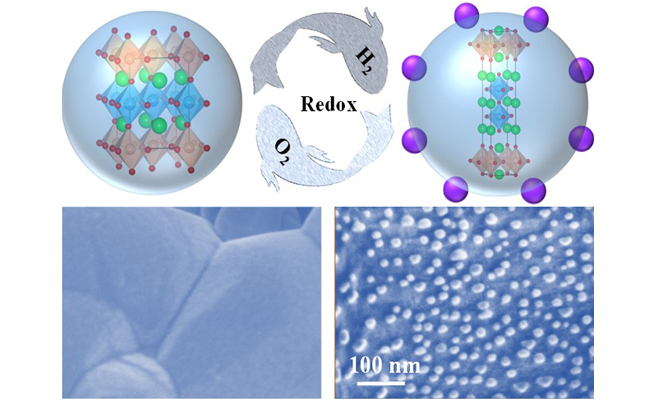 01 21, 2020Scientists revealed reversible exsolution/dissolution mechanism of perovskite electrode in solid oxide electrolysis cell for CO2 electrolysisScientists recently revealed the reversible exsolution/dissolution mechanism of CoFe alloy nanoparticles in Co-doped Sr2Fe1.5Mo0.5O6-δ perovskite cathode in solid oxide electrolysis cell (SOEC) for CO2 electrolysis. The results were published in Advanced Materials.Research team of Prof. WANG Guoxiong and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences recently revealed the reversible exsolution/dissolution mechanism of CoFe alloy nanoparticles in Co-doped Sr2Fe1.5Mo0.5O6-δ perovskite cathode in solid oxide electrolysis cell (SOEC) for CO2 electrolysis. The results were published in Advanced Materials. Reversible Exsolution/Dissolution of CoFe Alloy Nanoparticles in Co-doped Sr2Fe1.5Mo0.5O6-δ Cathode for CO2 Electrolysis (by LV Houfu) SOECs are able to convert CO2 and H2O to syngas, hydrocarbon fuel at cathode, while producing pure oxygen at anode. SOECs have the advantages of possessing solid and modular structure, fast reaction kinetics, high energy efficiency and low cost. Therefore, SOECs have promising applications in CO2 conversion and surplus renewable electricity storage. Perovskites have been extensively investigated as the most promising cathode materials for direct CO2 electrolysis in SOEC in the absence of protective gas, but many of them still suffer from insufficient CO2 electrolysis performance. In situ exsolving metal nanoparticles on the surface of perovskite have been explored as an efficient strategy to improve CO2 electrolysis performance, where abundant metal-oxide interfaces are generated for highly efficient CO2 electrolysis. Generally, reversible exsolution/dissolution of metal nanoparticles in perovskite have been proposed as vital properties for resolving the possible particle agglomeration and coke formation to enhance the stability. Until now, although some perovskites have demonstrated redox reversibility with exsolution and dissolution of metal nanoparticles in reducing and oxidizing atmosphere, fundamental understanding of these phenomena is still scarce and needs to be further investigated. In this recently published paper, they take advantage of in situ X-ray diffraction, in situ scanning transmission electron microscopy, environmental scanning electron microscopy and density functional theory calculations to investigate the exsolution/dissolution mechanism of CoFe alloy nanoparticles in Sr2Fe1.35Mo0.45Co0.2O6-δ (SFMC) double perovskite. Under reducing atmosphere, the facile exsolution of metallic Co promotes the reduction of Fe cation to generate CoFe alloy nanoparticles in SFMC, accompanying with the structure transformation from double perovskite to layered perovskite. Under oxidizing atmosphere, the spherical CoFe alloy nanoparticles are firstly oxidized to flat CoFeOx nanosheet, and then dissolved into the bulk accompanying with structure transformation from layered perovskite back to double perovskite. After reduction, metal-oxide interfaces between the exsolved CoFe nanoparticles and SFMC substrate with oxygen vacancies are constructed, which shows enhanced CO2RR performance and high stability. The CO2 electrolysis performance can be retrieved after 12 redox cycles due to the renewability of CoFe nanoparticles. This work was supported by the National Natural Science Foundation of China the National Key R&D Program of China and DICP. (Text and Image by LV Houfu and LIN Le)
01 21, 2020Scientists revealed reversible exsolution/dissolution mechanism of perovskite electrode in solid oxide electrolysis cell for CO2 electrolysisScientists recently revealed the reversible exsolution/dissolution mechanism of CoFe alloy nanoparticles in Co-doped Sr2Fe1.5Mo0.5O6-δ perovskite cathode in solid oxide electrolysis cell (SOEC) for CO2 electrolysis. The results were published in Advanced Materials.Research team of Prof. WANG Guoxiong and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences recently revealed the reversible exsolution/dissolution mechanism of CoFe alloy nanoparticles in Co-doped Sr2Fe1.5Mo0.5O6-δ perovskite cathode in solid oxide electrolysis cell (SOEC) for CO2 electrolysis. The results were published in Advanced Materials. Reversible Exsolution/Dissolution of CoFe Alloy Nanoparticles in Co-doped Sr2Fe1.5Mo0.5O6-δ Cathode for CO2 Electrolysis (by LV Houfu) SOECs are able to convert CO2 and H2O to syngas, hydrocarbon fuel at cathode, while producing pure oxygen at anode. SOECs have the advantages of possessing solid and modular structure, fast reaction kinetics, high energy efficiency and low cost. Therefore, SOECs have promising applications in CO2 conversion and surplus renewable electricity storage. Perovskites have been extensively investigated as the most promising cathode materials for direct CO2 electrolysis in SOEC in the absence of protective gas, but many of them still suffer from insufficient CO2 electrolysis performance. In situ exsolving metal nanoparticles on the surface of perovskite have been explored as an efficient strategy to improve CO2 electrolysis performance, where abundant metal-oxide interfaces are generated for highly efficient CO2 electrolysis. Generally, reversible exsolution/dissolution of metal nanoparticles in perovskite have been proposed as vital properties for resolving the possible particle agglomeration and coke formation to enhance the stability. Until now, although some perovskites have demonstrated redox reversibility with exsolution and dissolution of metal nanoparticles in reducing and oxidizing atmosphere, fundamental understanding of these phenomena is still scarce and needs to be further investigated. In this recently published paper, they take advantage of in situ X-ray diffraction, in situ scanning transmission electron microscopy, environmental scanning electron microscopy and density functional theory calculations to investigate the exsolution/dissolution mechanism of CoFe alloy nanoparticles in Sr2Fe1.35Mo0.45Co0.2O6-δ (SFMC) double perovskite. Under reducing atmosphere, the facile exsolution of metallic Co promotes the reduction of Fe cation to generate CoFe alloy nanoparticles in SFMC, accompanying with the structure transformation from double perovskite to layered perovskite. Under oxidizing atmosphere, the spherical CoFe alloy nanoparticles are firstly oxidized to flat CoFeOx nanosheet, and then dissolved into the bulk accompanying with structure transformation from layered perovskite back to double perovskite. After reduction, metal-oxide interfaces between the exsolved CoFe nanoparticles and SFMC substrate with oxygen vacancies are constructed, which shows enhanced CO2RR performance and high stability. The CO2 electrolysis performance can be retrieved after 12 redox cycles due to the renewability of CoFe nanoparticles. This work was supported by the National Natural Science Foundation of China the National Key R&D Program of China and DICP. (Text and Image by LV Houfu and LIN Le) -
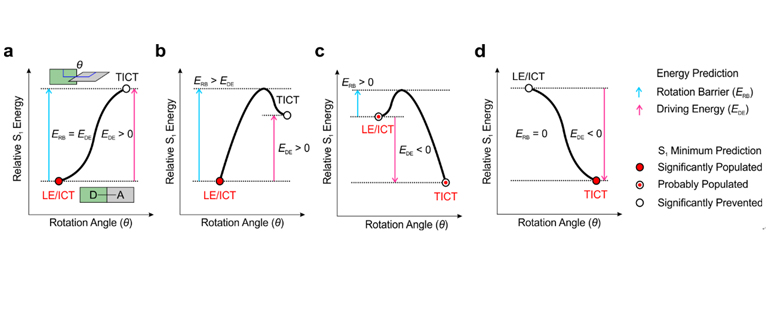 01 20, 2020Scientists Developed A Reliable Prediction Method for Twisted Intramolecular Charge Transfer (TICT) Formations in Fluorescent DyesScientists recently reported a reliable prediction method for twisted intramolecular charge transfer (TICT) formations in various fluorophores. Their results were published on Angew. Chem. Int. Ed.Research group led by prof. XU Zhaochao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, as well as LIU Xiaogang’s group from Singapore University of Technology and Design have recently reported a reliable prediction method for twisted intramolecular charge transfer (TICT) formations in various fluorophores. Their results were published on Angew. Chem. Int. Ed. TICT is a general photophysical process that can significantly quench the fluorescence and reduce the photo-stability of a dye. During such process, the donor or acceptor fragment will twist towards a perpendicular molecular conformation, resulting in a non-emissive and completely charge-separated species. Therefore, the suppression of TICT can greatly enhance the fluorescence intensity and photo-stability, meeting the requirements of modern single molecular biosensing and super resolution bioimaging. However, it is still challenging to accurately predict the TICT formations in various systems towards the quantitative design of bright and stable fluorophores. Researchers aim to deeply understand and explore the unique fluorescence mechanisms by combining experiments and calculations. Based on the preliminary mechanistic understanding (J.Am. Chem. Soc., 2016, 138, 6960-6963; Angew. Chem. Int. Ed., 2019, 58, 7073-7077), they realized accurate predictions in various TICT-related fluorophores. According to the nature of TICT formation, researchers summarized S1 potential energy surfaces (PESs) of 13 types of fluorophores. They found the rotation barrier (ERB) and driving energy (EDE) is the key in describing TICT formations. In particular, the fluorophore is more likely to stay in its bright state (LE or ICT states) with the larger ERB and EDE; If ERB > 0 and EDE < 0, the fluorophore will partly form TICT states; If ERB = 0 and EDE < 0, the fluorophore will significantly form TICT states, resulting in the substantial quenching of fluorescence. In short, the TICT formations can be accurately predicted via ERB and EDE.
01 20, 2020Scientists Developed A Reliable Prediction Method for Twisted Intramolecular Charge Transfer (TICT) Formations in Fluorescent DyesScientists recently reported a reliable prediction method for twisted intramolecular charge transfer (TICT) formations in various fluorophores. Their results were published on Angew. Chem. Int. Ed.Research group led by prof. XU Zhaochao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, as well as LIU Xiaogang’s group from Singapore University of Technology and Design have recently reported a reliable prediction method for twisted intramolecular charge transfer (TICT) formations in various fluorophores. Their results were published on Angew. Chem. Int. Ed. TICT is a general photophysical process that can significantly quench the fluorescence and reduce the photo-stability of a dye. During such process, the donor or acceptor fragment will twist towards a perpendicular molecular conformation, resulting in a non-emissive and completely charge-separated species. Therefore, the suppression of TICT can greatly enhance the fluorescence intensity and photo-stability, meeting the requirements of modern single molecular biosensing and super resolution bioimaging. However, it is still challenging to accurately predict the TICT formations in various systems towards the quantitative design of bright and stable fluorophores. Researchers aim to deeply understand and explore the unique fluorescence mechanisms by combining experiments and calculations. Based on the preliminary mechanistic understanding (J.Am. Chem. Soc., 2016, 138, 6960-6963; Angew. Chem. Int. Ed., 2019, 58, 7073-7077), they realized accurate predictions in various TICT-related fluorophores. According to the nature of TICT formation, researchers summarized S1 potential energy surfaces (PESs) of 13 types of fluorophores. They found the rotation barrier (ERB) and driving energy (EDE) is the key in describing TICT formations. In particular, the fluorophore is more likely to stay in its bright state (LE or ICT states) with the larger ERB and EDE; If ERB > 0 and EDE < 0, the fluorophore will partly form TICT states; If ERB = 0 and EDE < 0, the fluorophore will significantly form TICT states, resulting in the substantial quenching of fluorescence. In short, the TICT formations can be accurately predicted via ERB and EDE.
Different types of S1 potential energy surface. (Image by WANG Chao) Then the researchers designed PRODAN derivatives and AIE functional materials to validate the TICT predictions. From the plot of S1 PES of PRODAN, they found that N-TICT will occur in water, while O-TICT will be prohibited. The comparison of S1 PES in different fluorophores suggest that the introduction of azetidine functional group in P4 can suppress TICT due to large ERB (0.38 eV) and small magnitude of EDE (-0.14 eV). The experiment indicates that the quantum yield of P4 (Φ = 0.38) is about twice of P2 (introduction of dimethylamino). The authors also validated the existence of TICT in both P2 and P4 with different degree of fluorescence quenching. In comparison to dimethylamino, the introduction of azetidine effectively suppresses the TICT states. They validated the reliability of prediction method and concluded the 20-years mechanistic debates in PRODAN: PRODAN forms TICT states in water resulting in the quenching of fluorescence (other quenching mechanism may also exist). This work is supported by National Natural Science Foundation of China and Chinese Academy of Sciences Special Research Assistant Project. (Text by WANG Chao ).