Research News
-
 04 26, 2021Efficient Vacuum Deposition Approach Improves Performance of Formamidine-based Perovskite Solar CellsThe researchers from DICP of CAS developed high-throughput large-area vacuum deposition for high-performance formamidine-based PSCs.
04 26, 2021Efficient Vacuum Deposition Approach Improves Performance of Formamidine-based Perovskite Solar CellsThe researchers from DICP of CAS developed high-throughput large-area vacuum deposition for high-performance formamidine-based PSCs.
Perovskite solar cells (PSCs), the third generation photovoltage technology, are obtaining more and more attention.
The power conversion efficiency of small area PSCs can reach over 25.5% by solution method. However, it is difficult to achieve uniform preparation in large area and high-throughput production by solution preparation technology, and the solvent residues also affect the stability of the devices.
Recently, a research team led by Prof. LIU Shengzhong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. FENG Jiangshan from Shaanxi Normal University, developed high-throughput large-area vacuum deposition for high-performance formamidine-based PSCs.
Their findings were published in Energy & Environmental Science on March 26.
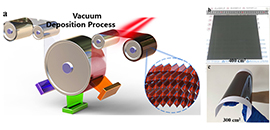
(a) Schematic illustration of multisource vacuum deposition with an in-vacuum annealing process for large-area perovskite films. Photographs of FA-based perovskite films deposited on (b) glass and (c) PET substrates (Image by DUAN Lianjie and WANG Hui)
The researchers prepared CsxFA1-xPbI3 thin films with large size, high density and high quality on 400cm2 rigid and 300cm2 flexible substrates by layer-to-layer deposition technology. By combing with low vacuum and low temperature annealing strategy, they effectively regulated the nucleation and grain growth of perovskite thin films.
"The photoelectric conversion efficiency (PCE) of the PSC fabricated by combining the Spiro-OMeTAD hole transport layer (HTL) is 21.32%, which is the highest PCE of the PSC fabricated by vacuum method ever reported," said Prof. LIU.
In addition, the researchers resolved the HTLs prepared by vacuum method, and obtained PCE of 18.89% by full vacuum deposition method. The efficiency of a PSC prepared by the all-vacuum process increases by 1% from its initial value after storage in ambient in the dark for 189 days, demonstration the high stability of devices prepared by full vacuum method.
This study shows that the all-vacuum method can realize the large-area and high-throughput preparation of PSCs with high efficiency and high stability. (Text by DUAN Lianjie and WANG Hui) -
 04 26, 2021New Findings on NaVPO4F Helps Designing High-performance Sodium Ion BatteriesThe researchers revealed the irreversible phase transition mechanism under variable temperature and sodium storage kinetics of monoclinic and tetragonal NaVPO4F.
04 26, 2021New Findings on NaVPO4F Helps Designing High-performance Sodium Ion BatteriesThe researchers revealed the irreversible phase transition mechanism under variable temperature and sodium storage kinetics of monoclinic and tetragonal NaVPO4F.
With the advantages of abundant resources, low cost and high safety, sodium ion batteries (SIBs) have broad application prospects. NaVPO4F is regarded a one of the most competitive cathodes in high-performance SIBs.
Two polymorphs with the tetragonal and monoclinic NaVPO4F have been reported. However, the accurate crystal structure, the transformation mechanism and the Na-storage dynamic of two-phase NaVPO4F have not been well studied, limiting further application of NaVPO4F in SIBs.
Recently, a research group led by Prof. LI Xianfeng and Prof. ZHENG Qiong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the irreversible phase transition mechanism under variable temperature and sodium storage kinetics of monoclinic and tetragonal NaVPO4F.
This study was published in Advanced Energy Materials on April 22.
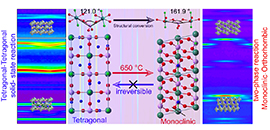
Phase transition mechanism and Na-storage kinetics of monoclinic and tetragonal NaVPO4F (Image by LING Moxiang)
The researchers obtained tetragonal and monoclinic NaVPO4F with high crystallinity and purity by low temperature hydrothermal and high temperature calcination.
"We first observed the accurate cell structure and two phase in atomic scale, the cell boundary of tetragonal phase is square, while that of monoclinic phase is parallelogram," said Prof. LI. "The NaVPO4F crystal undergoes an irreversible crystal conversion from tetragonal to monoclinic phase."
Moreover, they revealed the difference of binding energy caused by different V-P-V bond angles in tetragonal and monoclinic phases. Compared with tetragonal NaVPO4F, monoclinic phase exhibited better thermal stability due to its higher bond binding energy. As a result, the irreversible phase transition from tetragonal to monoclinic phase would occur when the temperature rises above 650 ℃.
The researchers also analyzed the Na-storage mechanism and electrochemical kinetics of the two crystal structures. They confirmed that the solid solution reaction process of tetragonal phase and "monoclinic to orthorhombic" two-phase transition reaction of monoclinic phase were in the electrochemical reaction, proving better electrochemical stability of tetragonal NaVPO4F.
In terms of kinetics, monoclinic NaVPO4F had higher intrinsic conductivity and sodium ion diffusion rate, showing higher power density, while the intrinsic Na-extraction activation energy of tetragonal NaVPO4F was higher, which was attributed to the higher working voltage and higher energy density.
As a result, monoclinic NaVPO4F could be employed as electrode in priority for high-power-density type SIBs, and tetragonal NaVPO4F could be used as the candidate electrode for high-energy-density type SIBs.
This study provides theoretical basis and technical support for the designing of high-performance sodium ion battery electrodes and the development of next generation sodium ion battery system with high-energy-density and high-power-density.
The above work was supported by the Strategic Priority Research Program of CAS, the National Natural Science Foundation of China, the DNL Cooperation Fund of CAS, and the Youth Innovation Promotion Association CAS. (Text by LING Moxiang) -
 04 20, 2021New Method Extends Cycle Life of Lithium AnodeScientists developed a paradigmatic N-rich polyether additive, nitrocellulose (NC), to stabilize the electrolytes for Li metal batteries.
04 20, 2021New Method Extends Cycle Life of Lithium AnodeScientists developed a paradigmatic N-rich polyether additive, nitrocellulose (NC), to stabilize the electrolytes for Li metal batteries.
Lithium (Li) metal is considered as the "Holy Grail" among the anode materials, due to its high theoretical capacity density and low electrochemical potential.
However, the cycle life of lithium metal batteries is normally limited by the repeated rupture of solid electrolyte interphase (SEI) that is always too brittle to withstand severe electrode interface deformation during cycling.
Recently, a research group led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a paradigmatic N-rich polyether additive, nitrocellulose (NC), to stabilize the electrolytes for Li metal batteries.
This study was published in Angewandte Chemie International Edition on March 10.
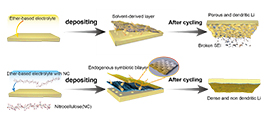
Illustration of the endogenous symbiotic Li3N/cellulose double SEI using nitrocellulose (Image by LUO Yang)
"We investigated the underlying reaction mechanism of lithium and NC by combing the detailed experimental characterizations and density functional theory (DFT) calculations," said Prof. LI.
The researchers found that the nitro group of NC could react with lithium metal, and the cellulose skeleton was tightly wrapped on the surface via Li-O bond. Therefore, the endogenous symbiotic Li3N/cellulose double SEI (ES-DSEI) was formed on the lithium surface almost simultaneously.
Moreover, they confirmed the structure and composition of the as-obtained bilayer by cryo-environmental transmission electron microscopy (ETEM) and X-ray photoelectron spectroscopy (XPS).
Lithium anode presented a dendrite-free morphology, which was detected by on-site optical microscopy and prolonged its cycle life due to the ES-DSEI. And the lithium metals with ES-DSEI were promising to be used in practical applications especially at elevated temperatures.
This work provides new insights on the exact role of additives at a fundamental level.
This work was supported by the National Natural Science Foundation of China and Youth Innovation Promotion Association of CAS. (Text by LUO Yang) -
 04 07, 2021Study Reveals Function and Molecular Mechanism of Metabolite Creatine in Cancer MetastasisResearchers found that creatine from both dietary uptake and de novo synthesis can promote the colorectal cancer metastasis to liver via activating SMAD2/3 in TGF-? signaling.
04 07, 2021Study Reveals Function and Molecular Mechanism of Metabolite Creatine in Cancer MetastasisResearchers found that creatine from both dietary uptake and de novo synthesis can promote the colorectal cancer metastasis to liver via activating SMAD2/3 in TGF-? signaling.
Cancer metastasis is the major cause of cancer-related death, and it is also the main challenge for cancer treatment.
Recently, a group led by Prof. PIAO Hailong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in cooperation with Prof. BU Pengcheng's group from the Institute of Biophysics of CAS and Prof. CHEN Gang's group from the 7th Medical Center of the PLA General Hospital, found that creatine from both dietary uptake and de novo synthesis can promote the colorectal cancer metastasis to liver via activating SMAD2/3 in TGF-β signaling.
This study was published in Cell Metabolism on April 2.
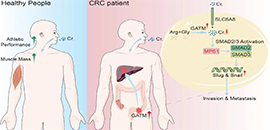
Creatine promotes cancer metastasis through activation of Smad2/3 (Image by WANG Wen, CHEN Di, and ZHANG Liwen)
The researchers established an in-situ mouse model of colorectal cancer to simulate the occurrence and development of colorectal cancer. They observed that supplementation of creatine could inhibit the growth of colorectal cancer in situ, but promote the metastasis of colorectal cancer and reduce the survival time of tumor-bearing mice.
Based on clinical tissues and mouse models, they further found that GATM, a rate-limiting enzyme for creatine biosynthesis, was highly expressed in liver metastatic colorectal cancer. Meanwhile, inhibition of GATM expression or activity could significantly inhibit the metastasis of colorectal cancer and prolong the survival time of tumor-bearing mice.
"The creatine could promote tumor cell invasion and metastasis by activating TGF-β downstream effector molecule SMAD2/3 and up-regulating the expression of SLUG/SNAIL," said Prof. PIAO.
This work was supported by National Natural Science Foundation of China. (Text by WANG Wen, CHEN Di, and ZHANG Liwen) -
 04 07, 2021Dual-bed Catalyst Enables High Conversion of Syngas to Gasoline-range Liquid Hydrocarbons
04 07, 2021Dual-bed Catalyst Enables High Conversion of Syngas to Gasoline-range Liquid Hydrocarbons
Gasoline, the primary transportation fuel, contains hydrocarbons with 5-11 carbons (C5-11) and is almost derived from petroleum at present.
Gasoline can also be produced from non-petroleum syngas. Nonetheless, achieving high conversions of syngas to C5-11 with excellent selectivity and stability remains a challenge.
A research group led by Prof. LIU Zhongmin and Prof. ZHU Wenliang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences realized highly efficient and selective conversion of syngas to gasoline-range liquid hydrocarbons over a dual-bed catalyst.
The study was published in Chem Catalysis on April 2.
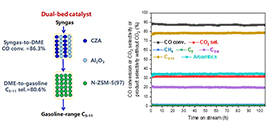
Schematic diagram for the conversion of syngas to gasoline-range liquid hydrocarbons over a dual-bed catalyst (CZA+Al2O3)/N-ZSM-5(97) and results of the stability test (Image by NI Youming)
This dual-bed catalyst, (CZA +Al2O3)/N-ZSM-5(97), consists of the conventional syngas-to-dimethyl ether catalyst CZA + Al2O3 in the upper bed and a dimethyl ether-to-gasoline catalyst N-ZSM-5(97) in the lower bed.
The selectivity of C5-11 and C3-11 in the hydrocarbon products reached 80.6% and 98.2%, respectively, along with 86.3% CO conversion.
The catalyst exhibited excellent stability, and the iso/n-paraffin ratio in the C5-11 products was up to 18. The nano-sized structure of N-ZSM-5(97) was beneficial for reducing coke and prolonging the lifetime; meanwhile, the low acid content of N-ZSM-5(97) was advantageous for increasing the C5-11 selectivity.
Compared with the Fischer-Tropsch synthesis process, this dual-bed syngas-to-gasoline (STG) process was more suitable for producing high-quality gasoline, along with the co-production of aromatic hydrocarbons.
This study was supported by the National Natural Science Foundation of China. (Text by NI Youming) -
 03 31, 2021A Tris-spiro Metalla-aromatic system Features Craig-Mobius AromaticityScientists synthesized and characterized a tris-spiroaromatic complex, hexalithio spiro vanadacycle.
03 31, 2021A Tris-spiro Metalla-aromatic system Features Craig-Mobius AromaticityScientists synthesized and characterized a tris-spiroaromatic complex, hexalithio spiro vanadacycle.
As aromaticity is one of the most fundamental concepts in chemistry, the construction of aromatic systems has long been an important subject.
Recently, Prof. YE Shengfa from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences, in collaboration with Prof. XI Zhenfeng from Peking University, synthesized and characterized a tris-spiroaromatic complex, hexalithio spiro vanadacycle.
Their study was published in nature communications on Feb 26.
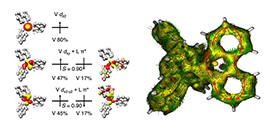
Structure and EPR/MOs/AICD of complex (Image by JIANG Yang)
In most conventional organic aromatic compounds, aromaticity stems from the high degree of delocalization of π-electrons. Compared with carbon atoms, the d orbitals of transition metals have multiple orientations and flexible bonding modes.
Consequently, the introduction of transition metals into aromatic systems may bring new metalla-aromatic structures and extend the concept of aromaticity.
In this study, the scientists analyzed the electronic structure using electron paramagnetic resonance coupled with density functional calculations.
They found that the complex possessed a doublet ground state, and the unpaired electron primarily resides in the V center. The XPS measurements detected the oxidation state of V to be higher than +3.
DFT calculations elucidated that the complex represented an unprecedented 40π Craig-Mobius aromatic system that was realized by electron delocalization of four electrons within the two V 3d orbitals and thirty-six electrons arising from the π* orbitals of the three biphenyl ligands.
This research showed the participation of d orbitals in transition metals played a key role, leading to the special metalla-aromatic structures that have been found for organic aromatic compounds.
This work presents a novel spiroaromatic system that may contribute to both aromaticity theory and organometallic chemistry. (Text by JIANG Yang)