As aromaticity is one of the most fundamental concepts in chemistry, the construction of aromatic systems has long been an important subject.
Recently, Prof. YE Shengfa from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences, in collaboration with Prof. XI Zhenfeng from Peking University, synthesized and characterized a tris-spiroaromatic complex, hexalithio spiro vanadacycle.
Their study was published in nature communications on Feb 26.
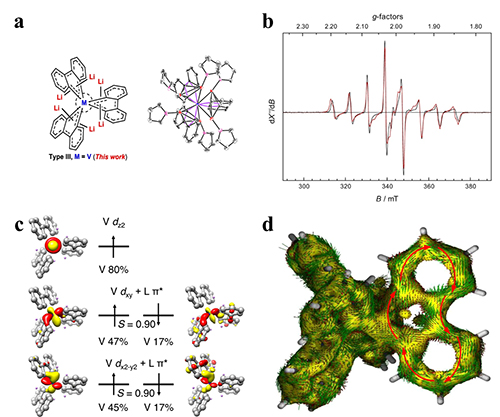
Structure and EPR/MOs/AICD of complex (Image by JIANG Yang)
In most conventional organic aromatic compounds, aromaticity stems from the high degree of delocalization of π-electrons. Compared with carbon atoms, the d orbitals of transition metals have multiple orientations and flexible bonding modes.
Consequently, the introduction of transition metals into aromatic systems may bring new metalla-aromatic structures and extend the concept of aromaticity.
In this study, the scientists analyzed the electronic structure using electron paramagnetic resonance coupled with density functional calculations.
They found that the complex possessed a doublet ground state, and the unpaired electron primarily resides in the V center. The XPS measurements detected the oxidation state of V to be higher than +3.
DFT calculations elucidated that the complex represented an unprecedented 40π Craig-Mobius aromatic system that was realized by electron delocalization of four electrons within the two V 3d orbitals and thirty-six electrons arising from the π* orbitals of the three biphenyl ligands.
This research showed the participation of d orbitals in transition metals played a key role, leading to the special metalla-aromatic structures that have been found for organic aromatic compounds.
This work presents a novel spiroaromatic system that may contribute to both aromaticity theory and organometallic chemistry. (Text by JIANG Yang)