Research News
-
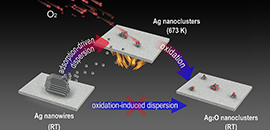 03 04, 2021Metallic State of Ag Nanoclusters in Oxidative Dispersion Identified in situScientists reported the oxygen adsorption-induced dispersion of metallic Ag nanoclusters in a typical oxidative atmosphere.Oxidative dispersion has been widely used in the regeneration of sintered metal catalysts as well as the fabrication of single-atom catalysts.The consensus on the oxidative dispersion process includes the formation of mobile metal oxide species from large metal particles and the capture of these species on a support surface. Nevertheless, the mechanism of oxidation-induced dispersion has yet to be confirmed via in situ electron microscopic and/or spectroscopic characterizations.Recently, a research team led by Prof. FU Qiang and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. YANG Bing from DICP and Prof. GAO Yi from the Shanghai Institute of Applied Physics of CAS, reported the oxygen adsorption-induced dispersion of metallic Ag nanoclusters in a typical oxidative atmosphere.The results were published in Nature Communications on March 3.Dynamic evolution of Ag nanostructures during oxidative dispersion (Image by LI Rongtan)By utilizing in situ imaging methods such as environmental scanning electron microscopy (ESEM), and newly developed near-ambient pressure photoemission electron microscopy (NAP-PEEM), researchers found that micron-scale Ag nanowires could be dispersed into subnanometer clusters under an oxygen atmosphere.Ex situ experiments indicated that Ag nanowires were converted into AgOx nanoclusters. Conversely, in situ near-ambient pressure photoelectron spectroscopy (NAP-XPS) directly demonstrated the presence of a transitional state of metallic Ag nanoclusters during dispersion at high temperatures, while the formation of the oxide occurred during the cooling process. The dynamic dispersion of Ag nanowires during CO oxidation was also demonstrated.Based on experimental and theoretical calculations, chemisorption of oxygen from the O2 atmosphere was shown to be the essential driving force for the dispersion of metallic Ag nanoclusters.This work provides a new understanding of the role of the O2 atmosphere in oxidative dispersion, which is particularly important for the prediction and control of the dynamic dispersion/redispersion of supported metal catalysts under similar reaction conditions.This work was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program (B) of the Chinese Academy of Sciences, the Ministry of Science and Technology of China, and the DNL Cooperation Fund. (Text by LI Rongtan)
03 04, 2021Metallic State of Ag Nanoclusters in Oxidative Dispersion Identified in situScientists reported the oxygen adsorption-induced dispersion of metallic Ag nanoclusters in a typical oxidative atmosphere.Oxidative dispersion has been widely used in the regeneration of sintered metal catalysts as well as the fabrication of single-atom catalysts.The consensus on the oxidative dispersion process includes the formation of mobile metal oxide species from large metal particles and the capture of these species on a support surface. Nevertheless, the mechanism of oxidation-induced dispersion has yet to be confirmed via in situ electron microscopic and/or spectroscopic characterizations.Recently, a research team led by Prof. FU Qiang and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. YANG Bing from DICP and Prof. GAO Yi from the Shanghai Institute of Applied Physics of CAS, reported the oxygen adsorption-induced dispersion of metallic Ag nanoclusters in a typical oxidative atmosphere.The results were published in Nature Communications on March 3.Dynamic evolution of Ag nanostructures during oxidative dispersion (Image by LI Rongtan)By utilizing in situ imaging methods such as environmental scanning electron microscopy (ESEM), and newly developed near-ambient pressure photoemission electron microscopy (NAP-PEEM), researchers found that micron-scale Ag nanowires could be dispersed into subnanometer clusters under an oxygen atmosphere.Ex situ experiments indicated that Ag nanowires were converted into AgOx nanoclusters. Conversely, in situ near-ambient pressure photoelectron spectroscopy (NAP-XPS) directly demonstrated the presence of a transitional state of metallic Ag nanoclusters during dispersion at high temperatures, while the formation of the oxide occurred during the cooling process. The dynamic dispersion of Ag nanowires during CO oxidation was also demonstrated.Based on experimental and theoretical calculations, chemisorption of oxygen from the O2 atmosphere was shown to be the essential driving force for the dispersion of metallic Ag nanoclusters.This work provides a new understanding of the role of the O2 atmosphere in oxidative dispersion, which is particularly important for the prediction and control of the dynamic dispersion/redispersion of supported metal catalysts under similar reaction conditions.This work was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program (B) of the Chinese Academy of Sciences, the Ministry of Science and Technology of China, and the DNL Cooperation Fund. (Text by LI Rongtan) -
 03 01, 2021Researchers Investigated Mechanism of Ni-Fe Based Catalysts in Electrocatalytic OER
03 01, 2021Researchers Investigated Mechanism of Ni-Fe Based Catalysts in Electrocatalytic OER
The research of electrocatalytic oxygen evolution materials is of great significance to the development of hydrogen energy and metal-air batteries. However, most of catalyst for commercialization are Ru, Ir-based and other noble metal catalysts.
At the same time, in-situ/operando Mossbauer spectroscopy was used to study the reaction mechanism of oxygen evolution reaction (OER) materials, in order to guide the synthesis of high-performance OER catalyst materials.
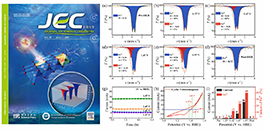
Recently, a group led by prof. WANG Junhu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with prof. HUANG Yanqiang from DICP, used the self-developed in-situ/operando electrochemical Mossbauer spectroscopy device to investigate the mechanism of Ni-Fe based catalysts in the electrocatalytic OER. They observed a large amount of Fe4+ near the onset potential of OER through experiments, and further confirmed that the current density of OER was positively associated with the content of high-valent iron species.
This study was published in the Journal of Energy Chemistry and it was selected as cover article.
Constructed nickel–iron (oxy)hydroxide with abundant in-situ produced high-valent iron species for efficient water oxidation (Image by KUANG Zhichong)
In this work, the researchers used Prussian blue analogues as precursors to prepare Ni-Fe oxyhydroxide with low crystallinity through topological transformation, which had a high OER activity. They discovered that a large amount of Fe4+ was formed near the OER starting potential by in-situ/operando electrochemical Mossbauer spectroscopy technology. As the voltage increased, its content could reach 40%.
"We first confirmed experimentally that the current density of OER was positively correlated with the content of high-valent iron species (Fe4+), and it deepened our understanding of the reaction mechanism of Ni-Fe oxyhydroxide in OER," said Prof. WANG.
This study is supported by the International Partnership Program of CAS. (Text by by KUANG Zhichong)
-
 02 26, 2021Scientists Probe Electronic Angular Momentum to a Chemical Reaction for the First TimeScientists developed molecular crossed beam apparatus with threshold ionization velocity map imaging technique, enabling to probe the scattering product with high angular resolution with quantum rotational-state recognition. With this powerful apparatus, in combined with new quantum reactive scattering theory, the electronic angular momentum effect to a chemical reaction was revealed for the first time.
02 26, 2021Scientists Probe Electronic Angular Momentum to a Chemical Reaction for the First TimeScientists developed molecular crossed beam apparatus with threshold ionization velocity map imaging technique, enabling to probe the scattering product with high angular resolution with quantum rotational-state recognition. With this powerful apparatus, in combined with new quantum reactive scattering theory, the electronic angular momentum effect to a chemical reaction was revealed for the first time.
A chemical reaction can be understood in detail at the quantum state-resolved level, through a combined study of molecular crossed beam experiments and theoretical quantum molecular reaction dynamics simulations.
At a single collision condition, the molecular crossed beam apparatus is able to detect the scattering angle-resolved product with rotational state-resolution. Whereas, with accurate global potential energy surface, quantum reactive scattering theory is able to predict the corresponding reactive scattering information.
In previous studies, the chemical reaction dynamics was revealed only with the product rotational state-resolution. And the investigation of a reaction at a finer level would be an inspiring break through.
Recently, Professor YANG Xueming from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and Professor WANG Xing'an from the University of Science and Technology of China developed molecular crossed beam apparatus with threshold ionization velocity map imaging technique, enabling to probe the scattering product with high angular resolution with quantum rotational-state recognition.
With this powerful apparatus, in combined with new quantum reactive scattering theory developed by Professor SUN Zhigang from the DICP, which included the electronic angular momentum effect, the electronic angular momentum effect to a chemical reaction was revealed for the first time.
This finding was published online in Science on Feb. 25, 2021.
The left circles are the experimental measurement of the product state-resolved differential cross sections of the F+HD reaction, the right image is the related partial-wave resonance wave function of the reaction (Image by WANG Ransheng)
There is distinguished reactive scattering quantum resonance in the F + HD (the Fluorine atom with the HD isotope of the H2 molecule) reaction. It has been taken as the prototype to resolve partial wave resonance structures in a chemical reaction.
With this feature, the scientists thought that the role of the electronic angular momentum of the F atom in this chemical reaction would be recognized. The F atom was characterized by p electronic orbit with l=1, which could influence the partial wave resonance structures.
It was found that, by including the electronic angular momentum, the single partial wave structure would split into four-fold partial wave resonance structure, which was capable of varying the angular distributions of the chemical product.
The energy of the electronic angular momentum is much smaller than the rotational energy of a diatomic molecule (~ several tens wave number). Its influence to a chemical reaction is subtle and difficult to detect. (Text by WANG Ransheng) -
 02 11, 2021New Method Helps to Improve Self-powered Integrated Circuit Photodetection
02 11, 2021New Method Helps to Improve Self-powered Integrated Circuit Photodetection
Multiple-cation and mixed-halide (FAMACs) perovskites, which are formed by incorporating Cs/MA/Br ions into FAPbI3 perovskites, are promising in applications in high-efficiency photovoltaic and photoelectronic devices.
Phase segregation into yellow non-perovskite phase often occurs during the crystallization process, influencing charge carrier mobility and carrier recombination dynamics, and thereby deteriorating device performance.
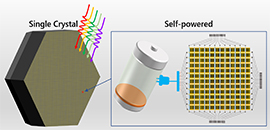
Recently, an international research team proposed an efficient strategy for obtaining high-quality perovskite single crystals with size up to five inches. The large and pure phase single crystals could be used to design high-performance self-powered integrated-circuit photodetectors.
Prof. LIU Shengzhong's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), Prof. LIU Yucheng from Shaanxi Normal University and Prof. Mercouri G. Kanatzidis from Northwestern University (Evanston) are involved in the study.
This work was published in Science Advances on Feb. 10.
Inch-sized high-quality perovskite single crystals formed by suppressing the phase segregation for applications in light-powered integrated circuits (Image by LIU Shengzhong)
The researchers selected a reducing agent (formic acid) for suppressing phase segregation during the crystallization process to obtain very large triple-cation mixed-halide perovskite single crystals.
This strategy yielded state-of-the-art perovskite single crystals with long carrier lifetime, high charge mobility, long carrier diffusion distance, superior uniformity, and long-term stability, thereby facilitating the design of high-performance self-powered integrated-circuit type photodetectors.
Moreover, since the photodetector comprising the crystal exhibited large responsivity, high photoconductive gain, excellent detectivity, and fast response speed, the researchers fabricated an integrated imaging system with uniform photo-response based on a 12 × 12 pixelated matrix of single crystal photodetectors.
"We believe that such a novel design will open new avenues for the applications of perovskite self-powered integrated circuits in devices relevant to imaging applications," said Prof. LIU. (Text by LIU Shengzhong) -
 02 08, 2021Multitasking MXene Inks Improve Performance of Printable Microelectrochemical Energy Storage DevicesResearchers developed aqueous printable multitasking MXene inks, which were applied as additive-free high-capacitance electrodes, sensitive pressure-sensing materials, highly conducting current collectors, metal-free interconnectors, and conductive binders.
02 08, 2021Multitasking MXene Inks Improve Performance of Printable Microelectrochemical Energy Storage DevicesResearchers developed aqueous printable multitasking MXene inks, which were applied as additive-free high-capacitance electrodes, sensitive pressure-sensing materials, highly conducting current collectors, metal-free interconnectors, and conductive binders.
The full integration of miniaturized energy harvesters, energy storage and energy consuming devices into a self-powered integrated micro-system on a single substrate is promising in flexible electronics applications.
Printing techniques exhibited great potential for integrated circuits and targeted functional devices. Screen printing enables scaling with high throughput, but it faces the challenge of fabricating multitasking ink.
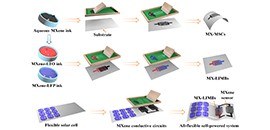
Recently, Prof. WU Zhongshuai's group and Prof. LIU Shengzhong's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed aqueous printable multitasking MXene inks, which were applied as additive-free high-capacitance electrodes, sensitive pressure-sensing materials, highly conducting current collectors, metal-free interconnectors, and conductive binders.
This study was published in Advanced Materials on Feb. 1.
Schematic of the fabrication of printable microelectrochemical energy storage devices and all-flexible self-powered integrated system (Image by ZHENG Shuanghao)
The researchers constructed MXene-based MSCs (MX-MSCs) and lithium ion micro-batteries (MX-LIMBs) via screen printing in a versatile and scalable manner on various substrates, including A4 paper, wood, fabric, SiOx-coated stainless steel, etc.
Notably, the MX-MSCs delivered ultrahigh areal capacitance of 1.1 F/cm2 and energy density of 13.8 μWh/cm2, both of which were much higher than most reported MX-MSCs. And a highly integrated MSCs pack consisting of 100 serially-connected MX-MSCs output a high voltage of 60 V, which were the highest value for MX-MSCs so far.
Furthermore, the quasi-solid-state MX-LIMBs, composed of only active materials and MXene, presented an increased areal energy density of 154 μWh/cm2.
The researchers also developed an all-flexible MXene-based self-powered integrated system, composed of a tandem thin-film silicon solar cell, a MX-LIMB, and a MXene hydrogel pressure sensor on a flexible co-planar substrate. And it was demonstrated that sensitively monitors of the system for the bending of body parts had a fast response of 35 ms.
This work was supported by National Natural Science Foundation of China, Dalian National Laboratory for Clean Energy of CAS, etc. (Text by ZHENG Shuanghao and HOU Xiaocheng) -
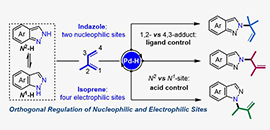 02 08, 2021Orthogonal Regulation Strategy Offers New Opportunities for Buildup of Molecular ComplexityResearchers developed an orthogonal regulation of nucleophilic and electrophilic sites in Pd-catalyzed regiodivergent couplings between indazoles and isoprene.
02 08, 2021Orthogonal Regulation Strategy Offers New Opportunities for Buildup of Molecular ComplexityResearchers developed an orthogonal regulation of nucleophilic and electrophilic sites in Pd-catalyzed regiodivergent couplings between indazoles and isoprene.
Dimethylallyl-related units play a significant role in enhancing lipophilicity of molecules and facilitating permeation across the cellular membrane.
Isoprene can serve as a precursor in the chemical synthesis of dimethylallyl-derived molecules. And indazoles are pharmacologically important scaffolds that found in numerous natural products and drugs.
Recently, a team led by Prof. CHEN Qing'an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in cooperation with Associate Professor JIANG Xuliang from Shenyang Pharmaceutical University, proposed a strategy that could realize orthogonal regulation of nucleophilic and electrophilic sites in Pd-catalyzed regiodivergent couplings between indazoles and isoprene.
Their findings were published in Angew. Chem. Int. Ed. on Jan. 18.
Pd-catalytic regiodivergent dimethylallylation of indazole with isoprene (Image by JIANG Wenshuang and JI Dingwei)
The researchers found that the 1,2-or 4,3-insertion pathway with respect to the electrophilic sites on isoprene could be controlled by the choice of ligands under Pd-hydride catalysis.
In terms of the nucleophilic sites on indazoles, the reaction occurred at either the N1-or N2-position of indazoles was governed by the acid co-catalysts.
This study not only contributes a practical tool for selective functionalization of isoprene, but also provides a guide to manipulate the regioselectivity for the N-functionalization of indazoles.
"This orthogonal regulation strategy of nucleophilic and electrophilic sites offers new opportunities for the buildup of molecular complexity," said Prof. CHEN.
This research was supported by the National Natural Science Foundation of China and Liaoning Revitalization Talents Program. (Text by JIANG Wenshuang and JI Dingwei)