Research News
-
 05 31, 2022Study Reveals Nature of Five-coordinated Aluminum on γ-Al2O3 SurfaceScientists for the first time observed the structure of Al(V) on the surface of γ-Al2O3 using ultrahigh-field (1.5GHz) solid-state Nuclear Magnetic Resonance (NMR) spectroscopy.
05 31, 2022Study Reveals Nature of Five-coordinated Aluminum on γ-Al2O3 SurfaceScientists for the first time observed the structure of Al(V) on the surface of γ-Al2O3 using ultrahigh-field (1.5GHz) solid-state Nuclear Magnetic Resonance (NMR) spectroscopy.
γ-Al2O3, an important catalyst and catalyst support, is widely used in various industrial applications. The five-coordinated aluminum, or Al(V), on the surface of γ-Al2O3, which is claimed as "super-five", can affect the catalytic performances of γ-Al2O3.
Recently, a research team led by Prof. HOU Guangjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Dr. GAN Zhehong from the National High Magnetic Field Laboratory, for the first time observed the structure of Al(V) on the surface of γ-Al2O3 using ultrahigh-field (1.5GHz) solid-state Nuclear Magnetic Resonance (NMR) spectroscopy.
This study was published in ACS Central Science and was selected as the Supplementary Cover Article on May 23.
Amorphous Al(V) domains on the surface of γ-Al2O3, liable to structural reconstruction and origins of distinct catalytic properties. (Image by WANG Rui and ZHAO Zhenchao)
The researchers investigated the structural properties of commercial γ-Al2O3 and amorphous alumina nanosheets (Al2O3-NS) rich in Al(V) by ultrahigh-field multinuclear and multi-dimensional Magic Angle Spinning (MAS) NMR.
They analyzed the aluminum species in both aluminas and found the flexible structural features on the surface of Al2O3-NS. And they demonstrated the hydroxyl groups on the surface of γ-Al2O3 with close spatial proximity that were able to be removed under high-temperature dehydration, resulting in surface structure reconstruction.
Moreover, by using ultrahigh-field 27Al-27Al double-quantum NMR, the researchers for the first time revealed that most Al(V) species tended to aggregate into Al(V) domains on the surface of γ-Al2O3 like Al2O3-NS, rather than tetragonal pyramid coordination on (100) surface previously predicted from theoretical models.
"These new insights into surface Al(V) species would help us to better understand the structure and function relationship of γ-Al2O3 when used as catalysts and catalyst supports", said Prof. HOU.
The study was supported by the National Natural Science Foundation of China, the National Key R&D Program, the Liaoning Revitalization Talents Program, and the Dalian Youth Science and Technology Star. (Text by WANG Rui and ZHAO Zhenchao) -
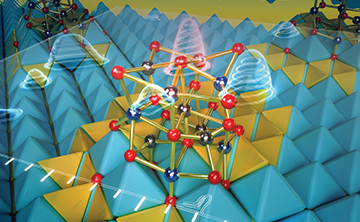
 05 30, 2022Researchers Discover Dynamic Transformation between Bilayer Islands and Dinuclear Clusters of Cr Oxide on Au(111) through Environment and Interface EffectsScientists discovered that the reversible dynamic conversion between Cr oxide (CrOx) nanoislands with the same thickness and CrOx clusters with the identical size supported on Au(111) surface under different redox treatments.
05 30, 2022Researchers Discover Dynamic Transformation between Bilayer Islands and Dinuclear Clusters of Cr Oxide on Au(111) through Environment and Interface EffectsScientists discovered that the reversible dynamic conversion between Cr oxide (CrOx) nanoislands with the same thickness and CrOx clusters with the identical size supported on Au(111) surface under different redox treatments.
Dynamic control of oxide nanostructures is crucial for the design of advanced oxide catalysts, which is also significant for understanding the active site and reaction mechanism in oxide catalysis.
Recently, a research team led by Prof. FU Qiang and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) discovered that the reversible dynamic conversion between Cr oxide (CrOx) nanoislands with the same thickness and CrOx clusters with the identical size supported on Au(111) surface under different redox treatments.
This work was published in Proceedings of the National Academy of Sciences of the United States of America on May 23.
Dynamic transformation between bilayer islands and dinuclear clusters of Cr oxide on Au(111) through environment and interface effects (Image by NING Yanxiao and YI Zhiyu)
The researchers grew two kinds of well-defined CrOx nanostructures on Au(111) and unambiguously identified them by scanning tunneling microscopy and theoretical calculations.
They found that the CrOx nanoislands featured a CrO2-bilayer (BL) structure consisting of two Cr2O3 monolayers bridged by one layer of O, and the CrOx clusters had a Cr2O7 stoichiometry. Moreover, oxidation treatment in O3 could disperse the CrO2-BL nanoislands into the Cr2O7-dinuclear clusters, which could be dynamically converted back to the CrO2-BL by annealing in an ultrahigh vacuum.
Furthermore, surface science experiments and theoretical simulations revealed that both surface oxygen atoms dissociated from O3 and the confinement effect of the Au substrate played important roles in the formation of the Cr2O7-dinuclear clusters.
"The oxide nanocatalysts with controlled size and structure can be stabilized by the specific environment and oxide-metal interface", Prof. FU said.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Liaoning Revitalization Talents Program. (Text/image by NING Yanxiao and YI Zhiyu) -
 05 20, 2022Novel Catalysts Promote Industrially Produce Clean Liquid Fuels from SyngasScientists developed the activated carbon-supported Co catalysts to produce clean liquid fuels from syngas with high selectivity. Using these catalysts, they have realized industrially synthesizing 150 kt/a of clean liquid fuels from syngas derived from coal in slurry-bed reactors in Yulin, China.
05 20, 2022Novel Catalysts Promote Industrially Produce Clean Liquid Fuels from SyngasScientists developed the activated carbon-supported Co catalysts to produce clean liquid fuels from syngas with high selectivity. Using these catalysts, they have realized industrially synthesizing 150 kt/a of clean liquid fuels from syngas derived from coal in slurry-bed reactors in Yulin, China.
China is rich in coal resources but has comparatively limited crude oil and natural gas. Therefore, conversion of coal to clean liquid fuels, which are mainly synthesized by crude oil, has received much attention in China.
A research group led by Prof. DING Yunjie and Prof. ZHU Hejun from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and their collaborators developed the activated carbon-supported Co catalysts to produce clean liquid fuels from syngas with high selectivity. Using these catalysts, they have realized industrially synthesizing 150 kt/a of clean liquid fuels from syngas derived from coal in slurry-bed reactors in Yulin, China.
The catalyst test showed that at the industrial facility's full load, the syngas' total conversion exceeded 98%, the selectivity of methane was less than 7%, and that of C3+ hydrocarbons/oxygenates exceeded 92%.
Fischer-Tropsch synthesis (FTS) is the most versatile process for converting syngas into various chemical products. Traditionally Co-based catalysts have poor selectivity of liquid which hinders their industrial applications.
The newly-developed Co-based catalysts were supported by carbon materials, leading to a high FTS activity, high C3+ selectivity, and good stability. Moreover, the catalysts could be recycled by the combustion method with no solid wastes.
"The direct conversion of syngas into clean liquid fuels using carbon material supported by a Co-based catalyst can be accomplished at an industrial scale in China, thereby creating more opportunities for clean and efficient use of coal resources," said Prof. DING. (Text by ZHAO Zi'ang)
The industrial facility of 150 kt/a of clean liquid fuels from syngas (Image by ZHAO Zi'ang) -
 05 16, 2022Heterogeneous Ethylene Hydroformylation Enables Highly-efficient Industrial Production of Propanal/n-PropanolResearchers has realized highly-efficient industrial production of propanal/n-propanol via heterogeneous ethylene hydroformylation.Olefins, hydrogen, and carbon monoxide can be converted to aldehydes by the hydroformylation of olefins. Upon further conversion, chemicals including alcohols, acids, and esters are obtained.However, commercial hydroformylation mainly employs homogeneous technology, which can cause problems including separation between catalyst and product, leaching of precious metals and ligands, the massive solvent issues, and the inefficient use of the heat from the exothermic reaction.Now, a research team led by Prof. DING Yunjie and Prof. YAN Li from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has realized highly-efficient industrial production of propanal/n-propanol via heterogeneous ethylene hydroformylation.Based on heterogeneous ethylene hydroformylation technology, the facility with propanal/n-propanol yield of 50 kt/year was put into operation in August 2020 in Ningbo, China. So far, it has been in stable operation for 22 months.The heterogeneous hydroformylation technology adopts porous organic polymers with large specific surface area and hierarchical porous structure as both carrier and ligand. It metalates rhodium ions to form single-Rh-sites catalyst with good performance and high stability using multiple Rh-P coordination bonds."The utilization rate of precious metals of this heterogeneous hydroformylation technology is nearly 100%, thereby making the leaching of precious metals and ligand negligible," said Prof. DING. "And there is no cost in the separation of catalyst and product in this process.""The reaction system is solvent-free, and the products have high purity. Moreover, a large amount of low-grade reaction heat can be efficiently used in the hydroformylation and hydrogenation reaction," said Prof. YAN. (Text by JIANG Miao)The industrial facility of 50 kt/year propanal/n-propanol by heterogeneous ethylene hydroformylation (Image by LI Cunyao)
05 16, 2022Heterogeneous Ethylene Hydroformylation Enables Highly-efficient Industrial Production of Propanal/n-PropanolResearchers has realized highly-efficient industrial production of propanal/n-propanol via heterogeneous ethylene hydroformylation.Olefins, hydrogen, and carbon monoxide can be converted to aldehydes by the hydroformylation of olefins. Upon further conversion, chemicals including alcohols, acids, and esters are obtained.However, commercial hydroformylation mainly employs homogeneous technology, which can cause problems including separation between catalyst and product, leaching of precious metals and ligands, the massive solvent issues, and the inefficient use of the heat from the exothermic reaction.Now, a research team led by Prof. DING Yunjie and Prof. YAN Li from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has realized highly-efficient industrial production of propanal/n-propanol via heterogeneous ethylene hydroformylation.Based on heterogeneous ethylene hydroformylation technology, the facility with propanal/n-propanol yield of 50 kt/year was put into operation in August 2020 in Ningbo, China. So far, it has been in stable operation for 22 months.The heterogeneous hydroformylation technology adopts porous organic polymers with large specific surface area and hierarchical porous structure as both carrier and ligand. It metalates rhodium ions to form single-Rh-sites catalyst with good performance and high stability using multiple Rh-P coordination bonds."The utilization rate of precious metals of this heterogeneous hydroformylation technology is nearly 100%, thereby making the leaching of precious metals and ligand negligible," said Prof. DING. "And there is no cost in the separation of catalyst and product in this process.""The reaction system is solvent-free, and the products have high purity. Moreover, a large amount of low-grade reaction heat can be efficiently used in the hydroformylation and hydrogenation reaction," said Prof. YAN. (Text by JIANG Miao)The industrial facility of 50 kt/year propanal/n-propanol by heterogeneous ethylene hydroformylation (Image by LI Cunyao) -
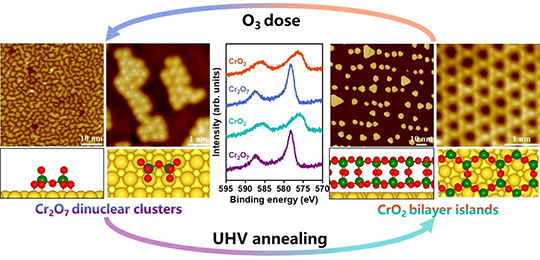
 05 12, 2022Novel Zeolites-silver Catalyst Boosts Formaldehyde Oxidation at Low TemperaturesScientists designed a tandem bifunctional zeolites-silver catalyst that boosted formaldehyde oxidation at low temperatures.
05 12, 2022Novel Zeolites-silver Catalyst Boosts Formaldehyde Oxidation at Low TemperaturesScientists designed a tandem bifunctional zeolites-silver catalyst that boosted formaldehyde oxidation at low temperatures.
A research group led by Prof. XIAO Jianping and Associate Prof. JIAO Feng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. QU Zhenping from Dalian University of Technology, designed a tandem bifunctional zeolites-silver (Ag) catalyst that could boost formaldehyde oxidation at low temperatures.
This study was published in Nature Communications on April 22.
Bi-functional zeolites-sliver catalyst enabled tandem oxidation of formaldehyde at low temperatures (Image by LI Na and DONG Xue)
Theoretical calculations and experimental results showed the activity of formaldehyde oxidation was influenced by the separation between two components in bifunctional catalyst.
The researchers found a volcano trend for the separation between ZSM-5 and Ag nanoparticles, which means it's not "the closer, the better".
Detached acidic ZSM-5 activated formaldehyde could generate gaseous intermediates of methyl formate, which was more easily oxidized by subsequent components (Ag). The Ag component would inevitably adsorb unreacted formaldehyde molecules and thereby led to lower methyl formate oxidation activity when the two components were packed too close.
The methyl formate oxidation on Ag components obtained high activity by suppressing the formation of dioxymethylene (DOM), which was hard to be further oxidized.
Compared to that of monofunctional supported silver catalyst, the formaldehyde conversion was increased by 50 times (100% versus 2%) at 70 °C.
The study was supported by the National Key Research and Development Program, and the National Natural Science Foundation of China. (Text by JIAO Feng) -
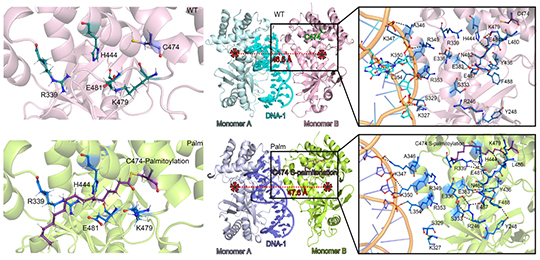 05 12, 2022Researchers Reveal Molecular Mechanism of ZDHHC18-mediated Palmitoylation of cGAS to Inhibit Innate ImmunityScientists revealed the molecular mechanism of ZDHHC18-mediated palmitoylation of cGAS to inhibit innate immunity.
05 12, 2022Researchers Reveal Molecular Mechanism of ZDHHC18-mediated Palmitoylation of cGAS to Inhibit Innate ImmunityScientists revealed the molecular mechanism of ZDHHC18-mediated palmitoylation of cGAS to inhibit innate immunity.
Cyclic GMP-AMP synthase (cGAS), a double-stranded DNA (dsDNA) sensing protein, plays an important biological function in the strong innate immune response induced by pathogen derived nucleic acids.
Previous studies have revealed the essential roles of cGAS in multiple biological processes, including pathogen invasion and autoimmune diseases. cGAS activity must be well regulated to maintain homeostasis, preventing both overinhibition and overactivation.
Recently, a research team led by Prof. LI Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. YIN Hang's group from Tsinghua University, revealed the molecular mechanism of ZDHHC18-mediated palmitoylation of cGAS to inhibit innate immunity.
This study was published in The EMBO Journal on April 19.
The researchers found that the palmitoyltransferase ZDHHC18 could inhibit the formation of 2:2 cGAS/DNA complex by palmitoylation of the C474 site of cGAS, thereby inhibiting the excessive activation of cGAS and negatively regulating cGAS biological activities.
Palmitoylation of the C474 site inhibits the dimerization of two 1:1 cGAS-DNA complexes (Image by LIU Ye)
"We found that palmitoylation of cGAS is a novel inhibitory mechanism of innate immune responses," said Prof LI. "It provides a potential target for drug development against viral infections and autoimmune diseases."
This work was supported by the National Natural Science Foundation of China. (Text by LIU Ye and CHU Huiying)