Pt-based electrocatalysts are promising for sluggish oxygen reduction reaction (ORR) in Polymer electrolyte membrane fuel cells (PEMFCs). However, the high cost and poor durability of Pt-based electrocatalysts hinder the commercialization of PEMFC.
Recently, a research group led by Prof. SUN Gongquan and Prof. WANG Suli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) fabricated a core-shell structure platinum-rhodium alloy (PtRh/Pt) electrocatalyst for oxygen reduction reaction with ultra-high stability and revealed its synergistic stabilization mechanism.
This study was published in ACS catalysis on Feb. 25.
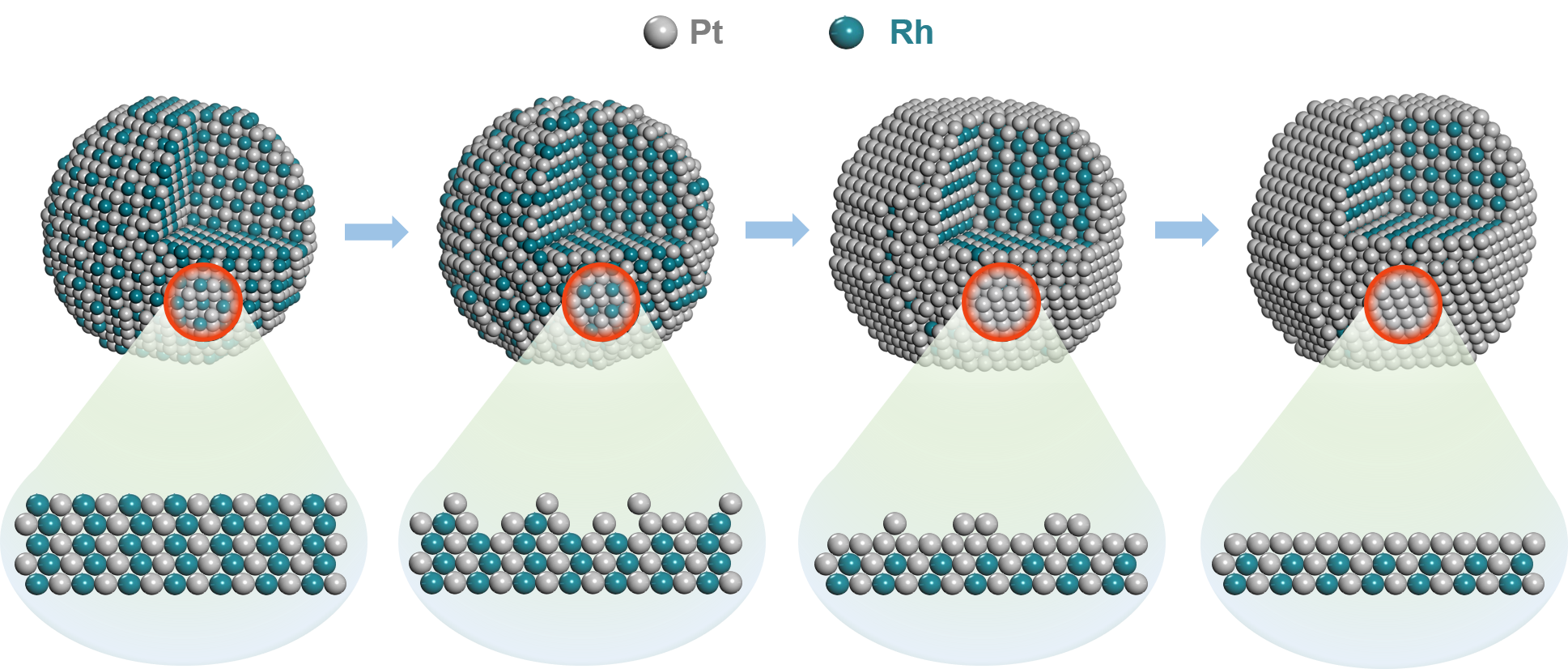
Schematic illustrations of the structure of the end of PtRh NDs during the 10K accelerated durability test cycles (Image by AN Zhao)
The researchers fabricated dendrite-like PtRh alloy catalyst by one-pot synthetic method, which underwent a dramatically structural evolution during the accelerated durability test. They found that the PtRh alloy catalyst could form a Pt-rich surface during the first 10K accelerated durability test cycles by the "self-healing" effect.
Moreover, they found that the segregation energy of rhodium atoms was higher than that of cobalt and nickel in other core-shell structures. Therefore, after 90,000 accelerated durability test cycles, the half-wave potential only shifted negatively by about 5 mV, and the mass activity remained 88%.
The stability of this PtRh/Pt catalyst was much higher than that of commercial platinum-carbon catalysts.
The study provides new opportunity for the practical design of durable Pt-based oxygen reduction reaction electrocatalysts for the commercialization of PEMFCs. (Text by AN Zhao)