Noble metals have incomparable advantages as special catalysts.
However, due to the influence of the relativistic effect, it's difficult to explain the specific structure and reaction mechanism of noble metals catalysts.
Recently, a research group led by Prof. JIANG Ling and Assoc. Prof. XIE Hua from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Assoc. Prof. LIU Zhiling's group from Shaanxi Normal University, provided a simple and effective mode for predicting the metal surfaces involved in catalytic process.
This study was published in The Journal of Physical Chemistry Letters on Dec. 23.
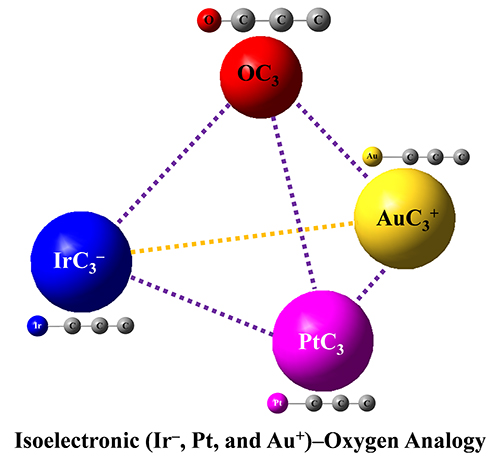
Isoelectronic IrC3–, PtC3 and AuC3+ Clusters Featuring the Structural and Bonding Resemblance to OC3 (Image by LIU Xuegang and XIE Hua)
The researchers investigated IrC3–, PtC3 clusters using photoelectron spectroscopy and density functional theory calculations.
They revealed that the IrC3–, PtC3, AuC3+ and OC3 complexes had analogous chemical bonding properties, which indicated that the metal atom could be viewed as oxygen atom in the isoelectronic species (IrC3–, PtC3, AuC3+) systems.
"Our finding broadened the notion of autogenic isolobality to the gas phase clusters that contain Ir–, Pt, Au+ and C centers," said Prof JIANG. "It provides a simple and effective empirical model for exploring the chemistry of noble mental by gas-phase chemistry," said Prof. JIANG.
This work was supported by the National Natural Science Foundation of China, the Youth Innovation Promotion Association of CAS, the Strategic Priority Research Program, International Partnership Program, and K. C. Wong Education Foundation. (Text by LIU Xuegang and XIE Hua)