Recently, the research team led by Prof. LIU Jianyong and Prof. HAN Keli from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences unraveled the synthetic mechanism of the novel energetic material of cyclo-N5- salt. Their results were published on The Journal of Physical Chemistry Letters.
The demand for novel high energy density materials (HEDMs) have been growing in the modern society. Compared with the traditional C, H, N, O based energetic materials, polynitrogens have higher chemical energy storage and no pollution, making them one of the most promising candidates for the novel high energy material.
In 2017, researchers first achieved the bulk-synthesis of cyclo-N5- salt from arylpentazole through the treatment of Fe(Gly)2 and m-CPBA. However, the production yield was low and the reaction mechanism was unknown, these restrict the application of cyclo-N5- as energetic material.
Given this, the research team led by Prof. LIU Liu and Prof.HAN Keli carried out an in-depth mechanistic study on the synthesis of cyclo-N5-. Firstly, they studied the synthesis of arylpentazole, which is the precursor of cyclo-N5-. The complete synthetic mechanism of arylpentazole was given, and the substituent effect was discussed, These results revealed the proper structure and reaction condition for arylpentazole production with higher yield.
Based on the results above, the present study unveiled the mechanism of selective C-N bond cleavage in arylpentazole. The results reveal that the high-spin state ferrous bisglycinate (Fe(Gly)2) is firstly oxidized by m-CPBA, leading to the formation of a high-valent iron(IV)-oxo complex. This Fe(IV)-oxo intermediate can effectively rupture the C-N bond in arylpentazole while keeping the pentazole ring intact.
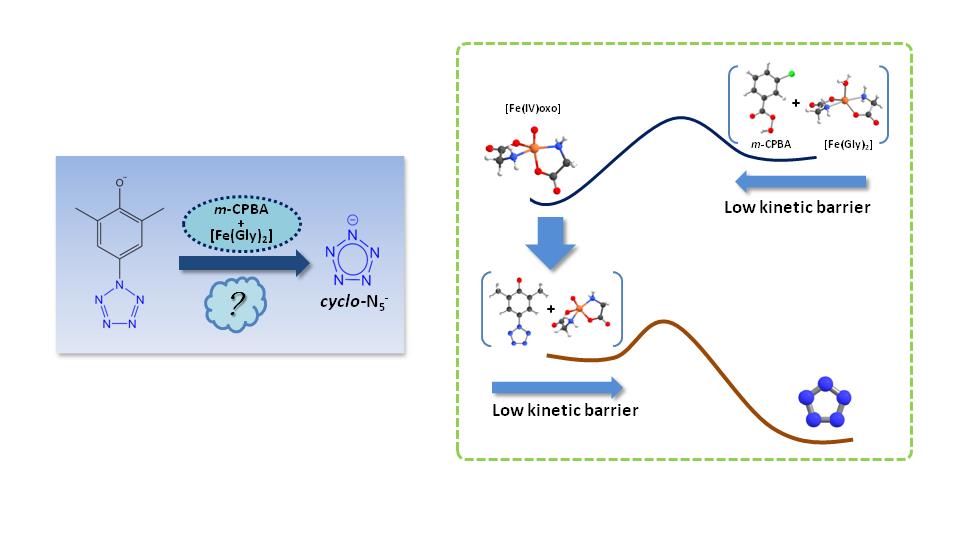
Besides, the π-π stacking effect between arylpentazole and m-CPBA promotes the formation of dimmers and trimers, which hinders the Fe-oxo from attacking the C-N bond of arylpentazole. How to effectively obtain iron(IV)-oxo structure is the key to increase the yield of cyclo-N5-. This study provides valuable theoretical guidance for the efficient synthesis of cyclo-N5-.
This research is supported by the Scientific Challenge Project and National Natural Science Foundation of China. (Text by SHANG Fangjian,LIU Runze)