Metalloenzyme can couple dioxygen activation to substrate functionalization, which often exhibits unrivalled efficiency even at ambient conditions.
It is believed that metal-superoxo intermediates are formed in dioxygen activation, and then further reduced to metal-peroxo and ultimately to -oxo species. However, the chemistry of metal-superoxo complexes remains largely unknown.
In their previous study, researchers led by Prof. YE Shengfa from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and their collaborators discovered that treating divalent Mn(II), Fe(II), and Co(II) model complexes and/or enzymes with dioxygen generated corresponding short-lived trivalent metal-superoxo intermediates. They could be transformed into trivalent metal-hydroperoxo species via H atom abstraction.
Recently, the scientists examined the reactions of a Mn(III)-superoxo complex with Bronsted and Lewis acids using resonance Raman, multi-frequency electron paramagnetic resonance spectroscopies coupled with quantum chemical calculations.
They revealed that the Mn(III)-superoxo complex underwent proton- and metal-coupled electron transfer to yield a Mn(IV)-hydroperoxo and a Mn(IV)-peroxo-Sc(III) species, respectively, thereby manifesting ambiphilic character of metal-superoxo complexes.
This unprecedented way to generate superoxo-to-peroxo conversion represented an important step toward better understanding of dioxygen activation and evolution processes occurring in metalloenzymes and artificial catalysts.
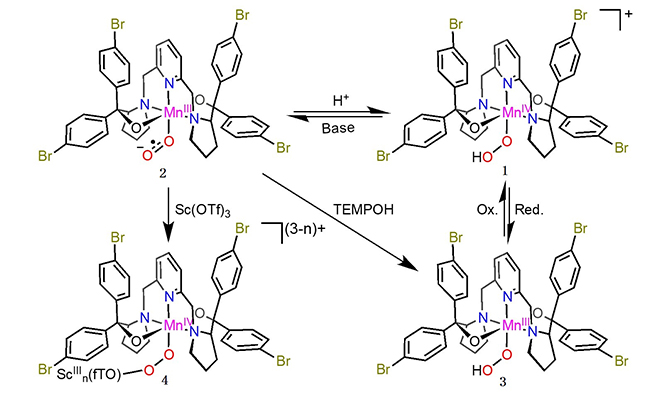
Reaction of 2 with 2-PPA, reversible interconversion of 1, 2, and 3, and formation of 4 (Image by JIANG Yang)
The study was published in J. Am. Chem. Soc. and it was supported by Special Support Program (A) of CAS. (Text by JIANG Yang)