Research News
-
 02 14, 2023Researchers Reveal Working Mechanism of Prussian Blue Cathode in Sodium-ion BatteryResearchers have revealed the charging/discharging mechanism and capacity degradation principle of Prussian blue cathode in SIBs through operando Mössbauer spectroscopy.
02 14, 2023Researchers Reveal Working Mechanism of Prussian Blue Cathode in Sodium-ion BatteryResearchers have revealed the charging/discharging mechanism and capacity degradation principle of Prussian blue cathode in SIBs through operando Mössbauer spectroscopy.
Prussian blue analogues (PBAs) are featured with high theoretical specific capacity, long cycle life, environmental benignity, and low cost. They are promising as cathode materials for sodium-ion batteries (SIBs). However, the working mechanism of PBAs is still unclear, hindering their applications.
Recently, a research team led by Prof. WANG Junhu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Xianfeng from DICP and Dr. Moulay Tahar Sougrati from the University of Montpellier, revealed the charging/discharging mechanism and capacity degradation principle of Prussian blue cathode in SIBs through operando Mössbauer spectroscopy.
This study was published in Nano Energy on Feb.5.
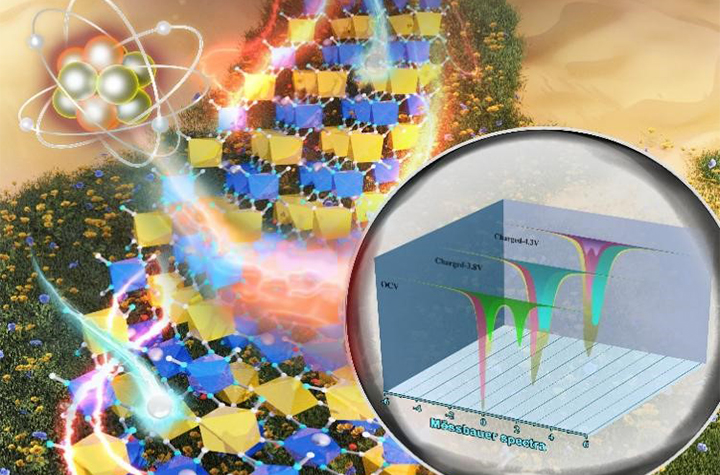
Schematic diagram of HS/LS Fe redox mechanism and local structure changes in the electrochemical reaction process (Image by WANG Zinan)
The researchers synthesized a sodium-rich Prussian blue by co-precipitation method, and used it as a cathode for SIB. The prepared SIB showed good electrochemical performance, which could maintain 81% capacity retention after 980 cycles under 120 mA g-1.
Furthermore, they on-line observed the working process of the SIB through operando Mössbauer spectroscopy, and revealed the working mechanism of Prussian blue using low-temperature 77K 57Fe Mössbauer spectroscopy, operando X-ray diffraction spectroscopy, and synchrotron X-ray absorption spectroscopy combined with the operando Mössbauer spectroscopy.
They found that the reaction of high-spin (HS) Fe and low-spin (LS) Fe reacted stepwise. The HS Fe reacted completely and contributed to most capacity, while only part of LS Fe reacted. This was the main reason for the discrepancy between practical capacity and theoretical one.
However, they also found that LS Fe could lead to more severe local distortions with respect to the effects imposed by the same amount of HS Fe, which decreased cycling stability.
Therefore, the good cycling performance for the Prussian blue cathode material could be assigned to the tiny changes of crystal parameters and the incomplete reaction of LS Fe.
"This work provides new insight into developing of PBAs cathode materials with high capacity and good cycling stability," said Prof. WANG.
This work was supported by the National Natural Science Foundation of China, the International Partnership Program of CAS, the President's International Fellowship Initiative (PIFI) of CAS. -
 02 13, 2023Researchers Reveal Sequential Oxidation Kinetics of Multi-cobalt Active Sites on Co3O4 Catalyst for Water OxidationResearchers have revealed sequential oxidation kinetics and determining roles of multi-cobalt active sites on Co3O4 catalyst for water oxidation.
02 13, 2023Researchers Reveal Sequential Oxidation Kinetics of Multi-cobalt Active Sites on Co3O4 Catalyst for Water OxidationResearchers have revealed sequential oxidation kinetics and determining roles of multi-cobalt active sites on Co3O4 catalyst for water oxidation.
Catalytic oxygen evolution reaction (OER), providing protons and electrons from water, plays an important role in clean energy storage and conversion processes. Its transformation requires sequential intermediate valence change steps, which makes the dynamics of the catalytical cycle complicated.
Such multi-redox mechanism involving multi-sites has implications to dictate the catalytic water oxidation. However, understanding the sequential dynamics of multi-steps in OER cycles on catalysts is still challenging.
Recently, a research group led by Profs. LI Can and WANG Xiuli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed sequential oxidation kinetics and determining roles of multi-cobalt active sites on Co3O4 catalyst for water oxidation.
The study was published in Journal of the American Chemical Society on February 01.
Schematic of the proposed valence change kinetics of OER catalytic cycle on Co3O4 (Image by KANG Wanchao)
The researchers employed quasi-operando transient absorption spectroscopy to study a typical photosensitization strategy, using Co3O4 nanoparticles as model catalysts.
They found that when OER initiated from fast oxidation of surface Co2+ ions, both surface Co2+ and Co3+ ions were active sites of the multi-cobalt centers for water oxidation.
In the sequential kinetics (Co2+ → Co3+ → Co4+), the key characteristic was fast oxidation and slow consumption for all the cobalt species. Due to this characteristic, the Co4+ intermediate distribution played a determining role in OER activity and results in the slow overall OER kinetics.
"Our study deepens the understanding of the multi-redox kinetics of multi-active sites in OER catalytic cycle. It also sheds light on the water oxidation mechanism and heterogeneous OER catalyst design," said Prof. Li.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the DICP Foundation of Innovative Research. -
 02 13, 2023Researchers Reveal Mechanism Regulating Rhodium Migration and Size Redistribution in Rhodium CatalystsResearchers have revealed a controlled-release mechanism to regulate Rh atom migration through two-dimensional zeolite nanosheets.
02 13, 2023Researchers Reveal Mechanism Regulating Rhodium Migration and Size Redistribution in Rhodium CatalystsResearchers have revealed a controlled-release mechanism to regulate Rh atom migration through two-dimensional zeolite nanosheets.
The migration of Rh atoms under a gas/reactive environment is important for the dynamic restructuring and size redistribution of Rh catalysts in a variety of structure-sensitive catalytic reactions.
Recently, a research group led by Prof. ZHANG Tao and Assoc. Prof. YANG Bing from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. ZHOU Si from the Dalian University of Technology, revealed a controlled-release mechanism to regulate Rh atom migration through two-dimensional (2D) zeolite nanosheets (ZSM-5-2D).
This study was published in ACS catalysis on January 5.
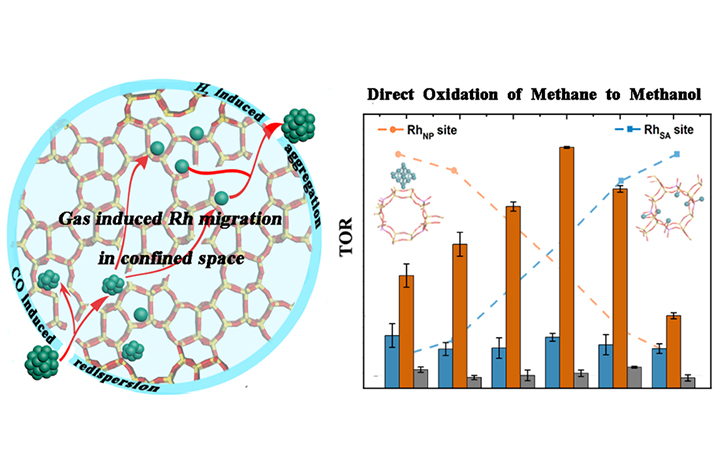
Dynamic aggregation/redispersion of Rh catalysts in/out of the ZSM-5-2D (Image by YANG Bing)
"The migration rate of Rh atoms can be precisely controlled by regulating the mutual interaction of the gas environment and support confinement, enabling a quasi-continuous size control over a wide range from single atom to nanoparticle reversibly," said YANG.
Through a variety of state-of-the-art in-situ characterizations, the researchers directly observed the dynamic aggregation/redispersion of Rh catalysts in/out of the ZSM-5-2D, enabling a quasi-continuous size control over a wide size range from Rh single atom to Rh nanoparticle reversibly.
The catalytic testing for mild oxidation of methane disclosed that the sub-nanometer Rh nanoclusters (RhNC) accounted for the highest methanol activity of 39.7 molCH3OOH·molRh-1·h-1 with a remarkable selectivity as high as 73.2%, far beyond that of single atom species and larger particles.
Density functional theory calculations and electron paramagnetic resonance spectroscopy further elucidated the size dependency by examining the formation of ·OH radicals for methane oxidation.
"This work reveals a controlled-release mechanism in zeolite encaged Rh catalyst, which is inspiring for the design of atomically precise catalysts," said YANG.
The above work was supported by the National Natural Science Foundation of China, the National Key R&D Program of China, the CAS Project for Young Scientists in Basic Research, and the Innovation Grant from DICP. -
 02 10, 2023Researchers Reveal New Competition Mechanism in Vacuum Ultraviolet Photoionization of DichloromethaneProf. LI Haiyang's group has proposed a new competition mechanism in vacuum ultraviolet photoionization of dichloromethane using a home-built time-of-flight mass spectrometer (TOFMS), which is helpful to study stratospheric ozone depletion mechanism and photodegradation of harmful haloalkanes.
02 10, 2023Researchers Reveal New Competition Mechanism in Vacuum Ultraviolet Photoionization of DichloromethaneProf. LI Haiyang's group has proposed a new competition mechanism in vacuum ultraviolet photoionization of dichloromethane using a home-built time-of-flight mass spectrometer (TOFMS), which is helpful to study stratospheric ozone depletion mechanism and photodegradation of harmful haloalkanes.
A research group led by Prof. LI Haiyang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed a new competition mechanism in vacuum ultraviolet photoionization of dichloromethane using a home-built time-of-flight mass spectrometer (TOFMS), which is helpful to study stratospheric ozone depletion mechanism and photodegradation of harmful haloalkanes.
The study was published in The Journal of Physical Chemistry Letters on Jan. 31.
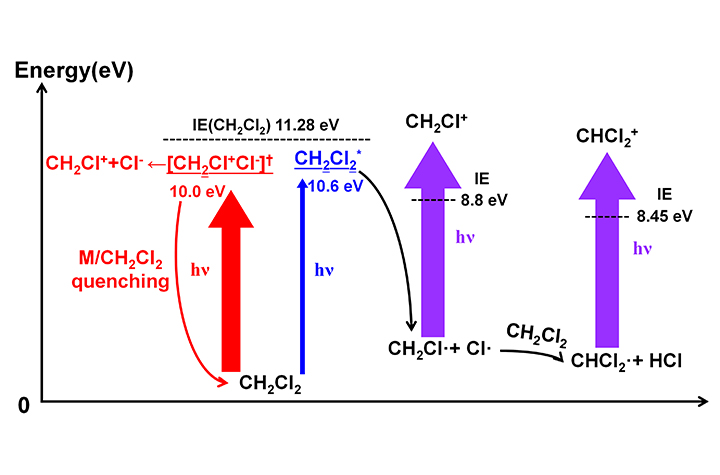
Proposed mechanism for the generation of CH2Cl+ and CHCl2+ when the neutral CH2Cl2 molecule was irradiated by a VUV Kr lamp (Image by YU Yi)
Dichloromethane (CH2Cl2) is widely used as industrial solvent, reaction medium in the pharmaceutical industry and feedstock for producing other chemicals. CH2Cl2 can cause environmental harm and health hazards due to its low boiling point and high volatility.
Strong presence of vacuum ultraviolet (VUV) light in the solar emission spectrum can induce the production of ozone-depleting Cl atom, therefore, the photochemistry of CH2Cl2 is crucial to stratospheric ozone chemistry.
In this study, the researchers have revealed the photoionization mechanism of CH2Cl2 under the irradiation of 10.0 and 10.6 eV light from a VUV krypton (Kr) lamp.
They demonstrated that CH2Cl+ was produced by two competitive channels: photoinduced ion-pair and photodissociation-assisted photoionization (PD-PI). The ion-pair channel was quenched efficiently at high number density of CH2Cl2, which reduced its contribution.
Moreover, they indicated that the dominant photodissociation channel of CH2Cl2 was CH2Cl2 + hν → CH2Cl· + Cl·, and the formed Cl· radical could further react with the CH2Cl2 molecule to form CHCl2· radical. Then CHCl2+ was generated by the photoionization of CHCl2·. Finally, they derived kinetic equations for the quantitative description of the production efficiencies of CH2Cl+ and CHCl2+.
"Our study enhances the overall understanding of the complicated photoexcitation behaviors of CH2Cl2 in the VUV regime, which helps to study the atmospheric photochemical process of haloalkanes and provides guidance for the photodegradation of hazardous haloalkanes," said Prof. LI.
"Our study proposed new insights into the complicated photoexcitation behaviors of CH2Cl2 in the VUV regime and revealed the important role of photodissociation in VUV photoionization at low photon flux," Prof. LI added.
This work was supported by the National Natural Science Foundation of China, the Scientific Instrument Developing Project of CAS, and DICP. -
 02 10, 2023New Strategy Enables Stepwise Photo-assisted Decomposition of Carbohydrates to HydrogenResearchers have proposed a "C-C bond-first" strategy and realized carbohydrates conversion into C1 liquid hydrogen carriers over Ta-CeO2 photocatalyst.
02 10, 2023New Strategy Enables Stepwise Photo-assisted Decomposition of Carbohydrates to HydrogenResearchers have proposed a "C-C bond-first" strategy and realized carbohydrates conversion into C1 liquid hydrogen carriers over Ta-CeO2 photocatalyst.
Hydrogen (H2), as a clean energy vector, can be produced via biomass photoreforming powered by solar light. For future biomass refining, biomass photoreforming deserves a high decomposition extent of biomass to maximize H2 production.
Recently, a research team led by Prof. WANG Feng, Dr. LUO Nengchao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Paolo Fornasiero from University of Trieste, proposed a "C-C bond-first" strategy and realized carbohydrates conversion into C1 liquid hydrogen carriers (LHCs, consisting of HCOOH and HCHO) over Ta-CeO2 photocatalyst. The LHCs could release H2 on site that was needed by either photo- or thermocatalysis.
This work was published in Joule on Jan. 31.
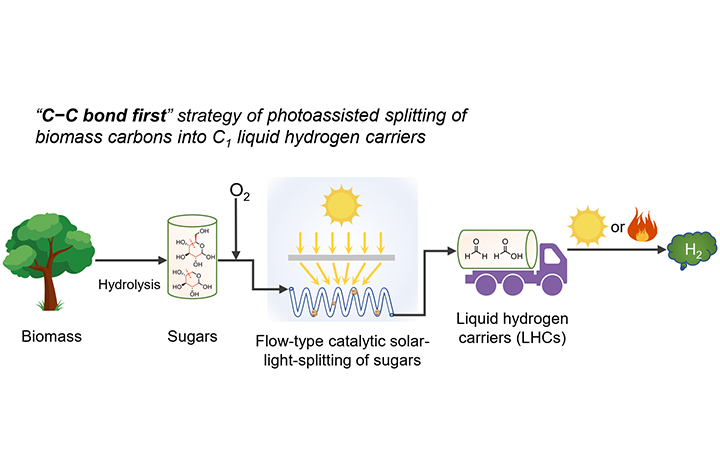
Illustration of a stepwise method for H2 production from biomass and storage in the form of C1 LHCs (Image by LUO Nengchao and REN Puning)
Currently, the main obstacle to high H2 yield is the far insufficient C-C bond breaking to convert biomass carbons into CO2 with maximization of H2 production.
In this study, the researchers demonstrated the significance of prioritized scission of C-C bonds in carbohydrates for photocatalytic hydrogen production and storage.
The proposed "C-C bond first" strategy emphasized prioritized biomass conversion to liquid C1 LHCs via fully breaking the C-C bonds. A synergistic Ta-CeO2 that utilized the photo- and thermal energy from solar light fully broke the C-C bonds of carbohydrates, producing C1 LHCs comprising HCOOH and HCHO with yields from 62% to 86%.
They found that during photocatalytic oxidation of carbohydrates, the elevated temperature was adopted to inhibit deleterious radical coupling over the strongly distorted Ta-CeO2. The resulting C1 LHCs that could be transported released only H2 and CO2, independent of solar light irradiation. The yield of H2 from glucose was 33%, much higher than that of direct photoreforming of glucose.
This stepwise method was also exemplified by flow-type photocatalytic oxidation of glucose under concentrated solar light, which enabled 15% yield of C1 LHCs from glucose via a cumulative irradiation time of 15.5 hours.
"This work provides a new perspective for H2 production and storage by emphasizing the significance of the prioritized scission of biomass C-C bonds," said LUO.
This work was supported by the Ministry of Science and Technology of the People's Republic of China, the National Natural Science Foundation of China, the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy, and the Italian Ministry of Foreign Affairs and International Cooperation-Project Italy-China n°. -
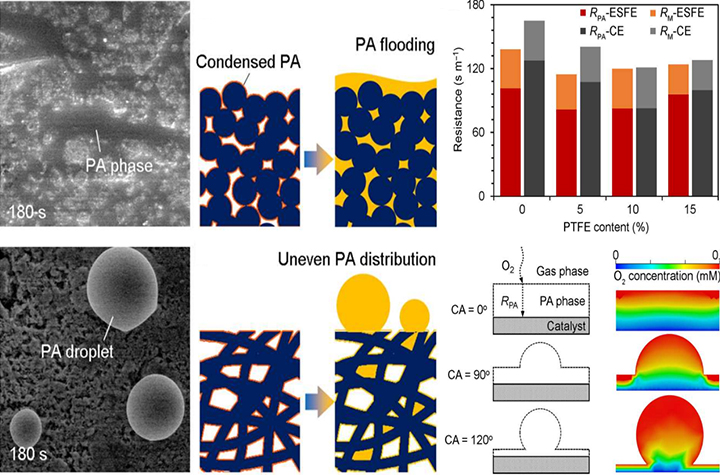 02 09, 2023Researchers Construct Uneven Phosphoric Acid Interfaces for Advanced High-temperature Polymer Electrolyte Fuel CellsResearchers constructed an uneven phosphoric acid interfaces within the nanofiber electrode for the high temperature polymer electrolyte fuel cells (HT-PEFCs), which reduce the resistance of oxygen transport.
02 09, 2023Researchers Construct Uneven Phosphoric Acid Interfaces for Advanced High-temperature Polymer Electrolyte Fuel CellsResearchers constructed an uneven phosphoric acid interfaces within the nanofiber electrode for the high temperature polymer electrolyte fuel cells (HT-PEFCs), which reduce the resistance of oxygen transport.
A research group led by Prof. WANG Suli and Prof. SUN Gongquan from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Science (CAS) constructed uneven phosphoric acid interfaces within the nanofiber electrode for the high temperature polymer electrolyte fuel cells (HT-PEFCs), which reduce the resistance of oxygen transport.
This study was published in Science Advances on January 25.
Manufacture, storage, and delivery of the highly purified hydrogen are challenges for the commercialization of PEFCs. Elevated temperature (>150oC) can realize PEFCs fed with hydrogen-rich product reformed from liquid fuels, which is expected to solve the problem of fuel storage and transportation.
However, insufficient performance with relatively inferior specific power limits the widespread application of HT-PEFCs. Different from the low temperature-PEFCs (LT-PEFCs), liquid phosphoric acid (PA) is adopted to form a proton-conductive phase and electrochemical interfaces within the porous electrodes of HT-PEFCs, leading to ultrahigh mass transport resistance and catalyst poisoning.
In order to reduce mass transport resistance for HT-PEFCs, the researchers designed uneven PA interfacial layers with dispersed droplets by constructing the fibrous electrode architecture. Due to the ultra-hydrophobic nature of the nanofiber networks, PA agglomeration occured as forms of non-infiltration droplets in the size of micrometer scale, which is different from the traditional immersion model of PA under working circumstances.
This uneven distribution of PA provided reduced thickness and coverage of the PA layers on the surface of catalyst within the porous electrode, leading to a 32% decrease in oxygen interfacial transport resistance and much enhanced electrochemical surface area compared to that of the conventional one.
As a result, the peak power density of the fibrous electrode reached 414 mW cm-2, 28% greater than that of the conventional porous electrode, demonstrating great application potential of HT-PEFCs.
This work was supported by the National Nature Science Foundation of China, the National Key Research and Development Program of China, the Foundation of the Key Laboratory of CAS, and DICP.