Embargo时间为12 January 2023 at 11:00 (US Eastern Time)
A research group led by Prof. BAO Xinhe, Prof. WANG Guoxiong and Prof. GAO Dunfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) recently revealed the coverage-driven selectivity switch from ethylene to acetate in high-rate CO2/CO electrolysis towards practical application.
The study was published in Nature Nanotechnology on January 12.
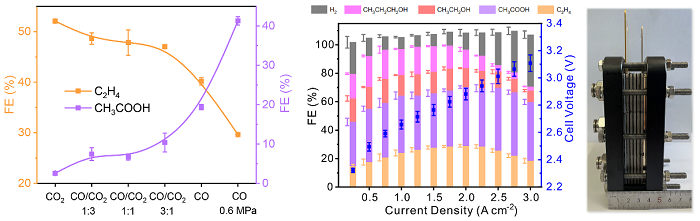
The performance of CO2/CO electrolysis and the photograph of electrolyzer stack (Image by GAO Dunfeng and WANG Guoxiong)
The CO2 electrolysis to valuable chemicals and fuels with power supply derived from renewable energy has emerged as an emerging carbon capture, utilization, and storage (CCUS) technology.
To push the CO2 electrolysis process towards practical application, achieving high selectivity of multicarbon (C2+) products at industrial current densities is highly desirable. In addition to rational catalyst design, tuning the microenvironments near catalyst surfaces has also been demonstrated to be effective in facilitating C–C coupling and improving C2+ production.
In this study, the researchers tuned the catalyst microenvironment using mixed CO/CO2 feeds which were typical composition of waste gases from steel plants and incomplete industrial combustion of fossil fuels. Specifically, they investigated CO/CO2 co-electrolysis over a nanoporous CuO nanosheet catalyst in an alkaline membrane electrode assembly (MEA) electrolyzer at high current densities.
They found that with increasing CO pressure in the feed, the major product gradually shifted from ethylene to acetate and the current density remarkably increased, up to 3.0 A cm-2 under 0.6 MPa pure CO feed.
Mechanistic studies indicated that the selectivity switch was induced by *CO coverage and local pH. Ethylene was preferentially generated at low *CO coverage, whereas acetate formation was favorable at high *CO coverage and high local pH.
Inspired by the mechanistic understanding, the researchers further improved electrolysis performance by optimizing electrolysis conditions. The Faradaic efficiency and partial current density of C2+ products reached 90.0% and 3.1 A cm-2, corresponding to a carbon selectivity of 100.0% and yield of 75.0%, outperforming thermocatalytic CO hydrogenation. The scale-up of the CO electrolysis process was demonstrated using an electrolyzer stack composed of four 100 cm2 MEAs, with the highest ethylene formation rate of 457.5 mL min-1 at 150 A and acetate formation rate of 2.97 g min-1 at 250 A.
"Our work highlights the promise of tuning catalyst microenvironments for the selective production of single C2+ products such as acetate and ethylene, and presents an effective scale-up demonstration of high-rate CO2/CO electrolysis towards practical application," said Prof. WANG.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Dalian National Laboratory for Clean Energy, the CAS, the Natural Science Foundation of Liaoning Province, the High-Level Talents Innovation Project of Dalian, and the Photon Science Center for Carbon Neutrality. (Text by GAO Dunfeng and WANG Guoxiong)