Research News
-
 01 23, 2016DICP Researchers constructe the Three-dimensional Structure of the Full-length Protein Through a Series of Molecular Modeling MeansBy using structural biological means such problems take years to be possible to complete, Molecular modeling is the only alternative in addition to the experiments. Guohui, through a series of molecular modeling means, constructed the three-dimensional structure of the full-length protein. By means of ultra-computing resources and ingenious design of simulation process, he completed the first work of two proteins of molecular recognition process power with 2 microseconds of simulation.
01 23, 2016DICP Researchers constructe the Three-dimensional Structure of the Full-length Protein Through a Series of Molecular Modeling MeansBy using structural biological means such problems take years to be possible to complete, Molecular modeling is the only alternative in addition to the experiments. Guohui, through a series of molecular modeling means, constructed the three-dimensional structure of the full-length protein. By means of ultra-computing resources and ingenious design of simulation process, he completed the first work of two proteins of molecular recognition process power with 2 microseconds of simulation.
Diseases are usually resulted from intracellular biological mutation or sharp changes in relative amounts of different components of molecules. And most experimental discovery is focused on the former.
AURKA is critical in cell differentiation, and also plays a vital role in tumor occurrence and development. Past experimental studies have shown that the nuclear AURKA is associated with poor cancer treatment, while, AURKA in cytoplasm does not always induce the formation of cancer, which can indicate that other than general kinase functions, AURKA also plays an unknown and important role. Our experimental collaborator Liu Qiang’s team in Sun Yat-sen's University found a significant increase of AURKA in breast cancer liver cells, and AURKA is formed by interaction with another protein RNPK complex with transcription factor function, activating the MYC promoter, and then control a series of changes related to protein expression in breast cancer. And how is this complex formed, what’s the three-dimensional structure, are essential to answer the above experimental phenomena of molecular mechanisms.
DICP Researchers constructe the Three-dimensional Structure of the Full-length Protein Through a Series of Molecular Modeling Means(Photo by LI Guohui)
By using structural biological means such problems take years to be possible to complete, Molecular modeling is the only alternative in addition to the experiments. Guohui, through a series of molecular modeling means, constructed the three-dimensional structure of the full-length protein. By means of ultra-computing resources and ingenious design of simulation process, he completed the first work of two proteins of molecular recognition process power with 2 microseconds of simulation. Statistical analysis found that the possible modes and their combined interaction sites, the theoretical prediction of binding sites 75% have been experimentally confirmed to be indeed affecting the binding of two proteins, thus providing an important theoretical basis to resolve this complex molecular recognition mechanism. His close work with the experimental workers completely answer microscopic molecular mechanisms of experimental findings. For the diagnosis and treatment of disease, this work provides a new research idea for the next generation of anti-cancer drug development by providing a new design target. Nature Communications 2016 (http://www.nature.com/ncomms/2016/160119/ncomms10180/full/ncomms10180.html).(Text/ Photo by LI Guohui)
-
 01 19, 2016Collaborative innovation forum of “Two Universities and One Institute” was held in Dalian Medical UniversityOn Jan.17th 2016, Dalian University of Technology (DLUT), Dalian Medical University (DLMU) and Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences held together the “Two Universities and One Institute” collaborative innovation forum in Dalian Medical University. 15 invited speakers from 3 institutions gave the plenary lectures. DICP faculty members including Profs. Guowang Xu, Lihua Zhang, Qingjian Hua, Renan Wu and Associate Prof. Guangbo Ge gave the presentations. About 200 participants attended this forum. More than 40 staffs and students from DICP participated.
01 19, 2016Collaborative innovation forum of “Two Universities and One Institute” was held in Dalian Medical UniversityOn Jan.17th 2016, Dalian University of Technology (DLUT), Dalian Medical University (DLMU) and Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences held together the “Two Universities and One Institute” collaborative innovation forum in Dalian Medical University. 15 invited speakers from 3 institutions gave the plenary lectures. DICP faculty members including Profs. Guowang Xu, Lihua Zhang, Qingjian Hua, Renan Wu and Associate Prof. Guangbo Ge gave the presentations. About 200 participants attended this forum. More than 40 staffs and students from DICP participated.
On Jan.17th 2016, Dalian University of Technology (DLUT), Dalian Medical University (DLMU) and Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences held together the “Two Universities and One Institute” collaborative innovation forum in Dalian Medical University. 15 invited speakers from 3 institutions gave the plenary lectures. DICP faculty members including Profs. Guowang Xu, Lihua Zhang, Qingjian Hua, Renan Wu and Associate Prof. Guangbo Ge gave the presentations. About 200 participants attended this forum. More than 40 staffs and students from DICP participated.
Collaborative innovation forum of “Two Universities and One Institute” was held in Dalian Medical University(Photo by Jian WANG)
After the forum, principal investigators from DCIP, DLUT and DLMU discussed how to make the cooperation in the field of precision medicine for cancer and chronic diseases to promote the translation of the bench work to clinic side.
Committee members decided DICP will hold next salon at end of March, 2016.(Text by Hai-long PIAO/ Photo by Jian WANG)
-
01 15, 2016DICP Researchers Firstly Discovered the Strong Metal-support Interaction (SMSI) Between Au Nanoparticles (NPs) and Hydroxyapatite (HAP) SupportLaboratory of Aerospace Catalysis and New Materials has recently made a new process on study of supported Au catalyst; they for the first time discovered the strong metal-support interaction (SMSI) between Au nanoparticles (NPs) and hydroxyapatite (HAP) support. This work is now online published in the Journal of the American Chemical Society
Laboratory of Aerospace Catalysis and New Materials has recently made a new process on study of supported Au catalyst; they for the first time discovered the strong metal-support interaction (SMSI) between Au nanoparticles (NPs) and hydroxyapatite (HAP) support. This work is now online published in the Journal of the American Chemical Society (J. Am. Chem. Soc. 2016, 138, 56-59, http://pubs.acs.org/doi/abs/10.1021/jacs.5b11306).
DICP Researchers Firstly Discovered the Strong Metal-support Interaction (SMSI) Between Au Nanoparticles (NPs) and Hydroxyapatite (HAP) Support (Photo by Hailian TANG and Botao QIAO)
Supported metal NPs catalysts are one of the most important types of heterogeneous catalysts. The SMSI is of great importance in determining the catalytic performance of supported catalysts. Therefore, new findings or new understanding of SMSI would be of great significance. Furthermore, SMSI may have potential application in improving the sintering resistance of metal NPs since the metal NPs were generally (partially) covered by a support layer in SMSI. This is of particular importance for supported Au catalysts which have demonstrated unique performance and shown potential application in many important chemical reactions while the major issues is the low stability of NPs in practical application.
In this work, the first example of SMSI between Au NPs and HAP, a nonoxide, was observed and demonstrated. The reversible encapsulation of Au NPs by HAP support, electron transfer, and changes in CO adsorption are identical to the classic SMSI except that the SMSI of Au/HAP occurred under oxidative condition; the opposite condition for the classical SMSI. The SMSI of Au/HAP not only enhanced the sintering resistance of Au NPs upon calcination but also improved their selectivity and reusability in liquid-phase reaction. It was found that the SMSI between Au and HAP is general and could be extended to other phosphate-supported Au systems such as Au/LaPO4. This new discovery may open a new way to design and develop highly stable supported Au catalysts with controllable activity and selectivity.
This work was financially supported by the National Natural Science Foundation of China. (Text/ Photo by Hailian TANG and Botao QIAO)
-
 01 13, 2016The 40th DICP Symposium on Oxide Surfaces Successfully Held in DICP
01 13, 2016The 40th DICP Symposium on Oxide Surfaces Successfully Held in DICP
The 40th DICP Symposium on Oxide Surfaces was held at DICP on Jan. 10-11, 2016. Prof. Xinhe Bao (DICP) and Prof. Qikun Xue (Tsinghua University), as co-chairs, organized this event. On behalf of DICP, Prof. Tao Zhang attended the opening ceremony and delivered the opening speech. This symposium has attracted more than 90 scientists from 12 countries around the world with 20 invited talks, 33 oral presentations and 20 posters.
The 40th DICP Symposium on Oxide Surfaces Successfully Held in DICP (Photo by Wansheng LIU)
Oxide surfaces play an important role in various scientific fields, including physics, chemistry, engineering, materials and biology. The scientific theme of this symposium focused on Surface and Interface, Two-dimensional Materials and Energy Conversion and Storage. Eight specific sessions were covered: 1. Structure and Theory; 2. Surface and Interface; 3. Catalysis; 4. Electrochemistry; 5. Two-dimensional Materials; 6.Novel Physical Phenomena; 7. Oxides on Metals; 8. Oxides under Ambient Pressure.
The two-day symposium has successfully promoted the in-depth collaboration between Chinese scientists and oversea researchers in oxide community. (Text by Peng Jiang, Photo by Wansheng Liu)
-
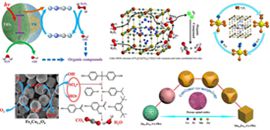 01 13, 2016DICP Researchers Made New Series of Progress in Mossbauer Study the Mechanism of Fenton-like Reaction
01 13, 2016DICP Researchers Made New Series of Progress in Mossbauer Study the Mechanism of Fenton-like Reaction
Nowadays, the scarcity of fresh water resources and the ever-growing environmental pollution have been attracting increased concern. The Fenton-like process has been widely investigated due to its high efficiency in removing persistent organic contaminants by in-situ production of SO4·- or HO· radicals. However, limited mechanism understanding hinders any significant advances in Fenton chemistry. In the past two years, Prof. Junhu Wang from the State Key Laboratory of Catalysis and the Mossbauer Effect Data Center has made a series of progress in investigating the mechanism of Fenton-like reaction, by using Mossbauer technique determine the coordination environment, Spin state and oxidation state of iron ions.
In 2014, the team developed a series of Prussian blue/TiO2 (PB/TiO2) nanocomposites as heterogeneous photo-Fenton catalyst to increase the FeII recovery in degrading organic contaminants in water. The Mossbauer spectra provided evidence that the excited electrons of TiO2 by UV irradiation could accelerate the cycle of low spin FeIII to FeII in PB and then led to the higher activity (Catal. Sci. Technol., 2015, 5, 504-514. doi: 10.1039/C4CY00947A). Then, two kinds of Fe-Co Prussian blue analogues (Fe-Co PBAs) with different iron valence state, Fe3[Co(CN)6]2·12H2O and Fe[Co(CN)6]·2H2O, were developed as novel photo-Fenton catalysts for in-depth investigation of the heterogeneous Fenton reaction mechanism. The efficient redox cycling of iron species in the Fe-Co PBAs photo-Fenton process was deeply explored by Mossbauer spectroscopy. The excellent photo-Fenton activities of these Fe-Co PBAs were ascribed to the existence of highly dispersed water coordinated iron sites and abundant vacancies in the metal-organic-frameworks. In collaboration with Prof. Hongxian Han from the State Key Laboratory of Catalysis, singlet oxygen (O1 2) produced indirectly from HOO· and HO· was found to participate directly in the degradation of RhB (Appl. Catal. B 2016, 181, 788-799. doi:10.1016/j.apcatb.2015.05.033 2015/07-09 Top 25 Hottest Articles: 25/25). To further study the reaction intermediates and pathway of active iron species during the Fe-Co PBA catalyzed Fenton-like process, hydrazine (Hz) was newly introduced to enhance the oxidation performance of the Fenton system. Combining the XPS and M?ssbauer spectroscopy results, the Hz coordinated iron site (H2NH2N-Fe), which is evolved from the original water coordinated iron site (H2O-Fe), was identified as a more active site which largely increased the reaction rate (Catal. Commun., 2016, doi: 10.1016/j.catcom.2016.01.004).
DICP Researchers find the Mechanism of Fenton-like Reaction by Using Mossbauer Technique (Photo by Xuning LI)
Based on the deeply understanding the Fenton-like reaction mechanism as well as the structural features of the Fe-Co PBAs, the team successfully prepared a series of porous FexCo3-xO4 nanocages via a facile strategy by heating FeyCo1-y-Co PBAs nanospheres with tunable size and morphology. The prepared FexCo3-xO4 nanocages were found to be efficient to activate PMS into radicals, which could further degraded BPA to small organic compounds or even inorganic carbon forms. Mossbauer and XPS techniques were applied to illustrate the catalytic mechanism and B-site CoII on the surface of FexCo3-xO4 nanocages was determined as the main factor for PMS activation (Appl. Catal. B 2016, 181, 788-799. doi:10.1016/j.apcatb.2015.08.050 2015/07-09 Top 25 Hottest Articles: 15/25). Meanwhile, the team has successfully synthesized series of MnyFe1-y-Co PBAs nano-dices with well-controlled morphology and composition through simply modulating the relative content of Mn. Based on the fact of that three series of MyFe1-y-Co PBAs (M = Co, Mn and Zn) with well-controlled morphology have been successfully prepared through this novel and scalable strategy, a “copolymer-co-morphology” conception was unprecedentedly proposed. In collaboration with Prof. Luhua Jang from Dalian National Laboratory for Clean Energy, the octahedral-site MnIII/MnIV content in MnxFe1.8-xCo1.2O4, mainly determined by sensitive 57Fe Mossbauer and XPS techniques, was discovered to be directly correlated with the oxygen reduction/evolution reaction (ORR/OER) activity (Nanoscale, 2016, doi: 10.1039/C5NR07193C).
The series of research not only opens a new way for the general use of PBAs in photo-Fenton process, but also gives a deeper insight into the heterogeneous Fenton reaction mechanism which is nowadays very limited. In addition, the “copolymer-co-morphology” conception will be beneficial for morphologically controlled syntheses of other types of CPs and spinel oxides.
The above-mentioned research work has been financially supported by the National Natural Science Foundation of China and the Chinese Academy of Sciences Visiting Professorships for Senior International Scientists. (Text/Photo by Xuning LI)
-
 01 08, 2016The Monodisperse Micron Silica Packing Production Line is Successfully OperatedThe monodisperse micron silica packing production line was successfully operated. This production line could manufacture narrow pore size distribution and high uniformed monodisperse mesoporous micron Silica, which researched and designed independently by the team of Prof. Shudong Wang and Dr. Hongjiu Su in the clean energy national laboratory. The particle size of 2 μm, 3.5 μm, 5 μm and 10μm microsilica sphere was successfully prepared as well as the particles size dimension between the 2 to 10 μm could be controllable synthesized. After a series of tests, the physical properties such as mechanical strength, homogeneity and spherical degree perfectly meet the requirements of chromatographic packing material.
01 08, 2016The Monodisperse Micron Silica Packing Production Line is Successfully OperatedThe monodisperse micron silica packing production line was successfully operated. This production line could manufacture narrow pore size distribution and high uniformed monodisperse mesoporous micron Silica, which researched and designed independently by the team of Prof. Shudong Wang and Dr. Hongjiu Su in the clean energy national laboratory. The particle size of 2 μm, 3.5 μm, 5 μm and 10μm microsilica sphere was successfully prepared as well as the particles size dimension between the 2 to 10 μm could be controllable synthesized. After a series of tests, the physical properties such as mechanical strength, homogeneity and spherical degree perfectly meet the requirements of chromatographic packing material.
The monodisperse micron silica packing production line was successfully operated. This production line could manufacture narrow pore size distribution and high uniformed monodisperse mesoporous micron Silica, which researched and designed independently by the team of Prof. Shudong Wang and Dr. Hongjiu Su in the clean energy national laboratory. The particle size of 2 μm, 3.5 μm, 5 μm and 10μm microsilica sphere was successfully prepared as well as the particles size dimension between the 2 to 10 μm could be controllable synthesized. After a series of tests, the physical properties such as mechanical strength, homogeneity and spherical degree perfectly meet the requirements of chromatographic packing material.
The Monodisperse Micron Silica Packing Production Line is Successfully Operated (Photo by Diannan GAO)
High performance liquid chromatographic (HPLC) is not only used in efficient analysis and detection technique, but also in separation field especially for traditional Chinese medicine, natural products, synthetic drugs and components etc. The core of HPLC technique is chromatographic column packing. Among today’s appropriate packing materials, silica accounted for 80%. Then, in the process of the preparation of silica filler, the bottleneck is how to control the characters of the packing material in pore size, concentration, micron scale, spherical degree and uniformity. However a few large foreign companies monopolized the high-end silica packing and domestic production is difficult to meet the requirements of HPLC. Therefore, the preparation of monodisperse silica micro silica gel technology greatly limits HPLC in the applications of analysis, detection and separation of drugs in China. Using a new chemical engineering method which based on the theory of existing catalyst design and synthesis, the team of Energy and Environment Engineering put forward the new technology of synthesis of the monodisperse micron silica packing and successfully synthesis the narrow pore size distribution and high uniformed monodisperse mesoporous micron silica packing. The micro silica packing on the column pressure, column efficiency and wide spectrum wholly meet the requirement of chromatographic packing materials. (Photo by Diannan GAO, Text by Hongjiu SU)