Laboratory of Aerospace Catalysis and New Materials has recently made a new process on study of supported Au catalyst; they for the first time discovered the strong metal-support interaction (SMSI) between Au nanoparticles (NPs) and hydroxyapatite (HAP) support. This work is now online published in the Journal of the American Chemical Society (J. Am. Chem. Soc. 2016, 138, 56-59, http://pubs.acs.org/doi/abs/10.1021/jacs.5b11306).
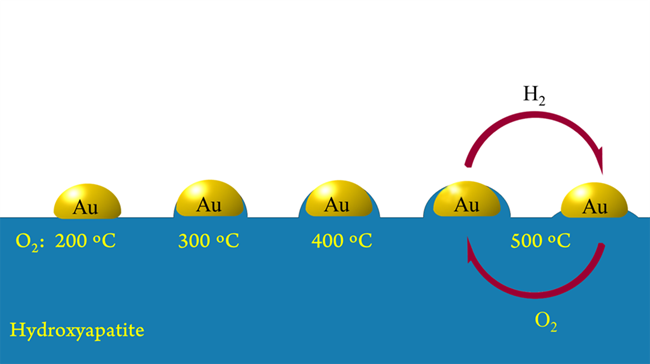
DICP Researchers Firstly Discovered the Strong Metal-support Interaction (SMSI) Between Au Nanoparticles (NPs) and Hydroxyapatite (HAP) Support (Photo by Hailian TANG and Botao QIAO)
Supported metal NPs catalysts are one of the most important types of heterogeneous catalysts. The SMSI is of great importance in determining the catalytic performance of supported catalysts. Therefore, new findings or new understanding of SMSI would be of great significance. Furthermore, SMSI may have potential application in improving the sintering resistance of metal NPs since the metal NPs were generally (partially) covered by a support layer in SMSI. This is of particular importance for supported Au catalysts which have demonstrated unique performance and shown potential application in many important chemical reactions while the major issues is the low stability of NPs in practical application.
In this work, the first example of SMSI between Au NPs and HAP, a nonoxide, was observed and demonstrated. The reversible encapsulation of Au NPs by HAP support, electron transfer, and changes in CO adsorption are identical to the classic SMSI except that the SMSI of Au/HAP occurred under oxidative condition; the opposite condition for the classical SMSI. The SMSI of Au/HAP not only enhanced the sintering resistance of Au NPs upon calcination but also improved their selectivity and reusability in liquid-phase reaction. It was found that the SMSI between Au and HAP is general and could be extended to other phosphate-supported Au systems such as Au/LaPO4. This new discovery may open a new way to design and develop highly stable supported Au catalysts with controllable activity and selectivity.
This work was financially supported by the National Natural Science Foundation of China. (Text/ Photo by Hailian TANG and Botao QIAO)