Carbon dioxide (CO2) electroreduction in solid oxide electrolysis cell (SOEC) is an effective way to combine CO2 conversion and renewable electrical energy storage. It's crucial to improve the catalytic activity cathode for the electroreduction in SOECs since CO2 molecule itself is relatively stable.
Au nanoparticles exhibit high catalytic activity in many reactions. However, they are rarely used as a cathode because nano Au prepared by solution methods is prone to sintering and loses catalytic activity at high temperatures.
Recently, a research group led by Prof. ZHU Xuefeng and Prof. YANG Weishen from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed a new method to in situ dispersed a Au layer into nanoparticles by applying a high activation voltage. The resultant Au electrode can be used for CO2 electrochemical reduction in SOECs at 800 °C.
This study was published in Nano Letters on August 6.
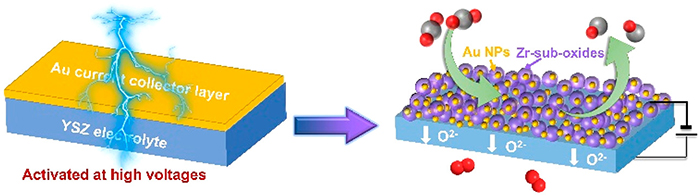
Nano Au-ZrOx cathode with high CO2 electroreduction activity was in-situ constructed from Au layer activated at high applied voltages (Image by ZHANG Lixiao)
The proposed method not only simplified the battery preparation process, but also increased the CO2 reduction current density by 38 times and reduced the polarization resistance by 75 times.
Combining in-situ near ambient pressure X-ray photoelectron spectroscopy (APXPS) and theoretic calculations, the researchers found that the strong interaction between Au and ZrOx promoted the formation and stabilization of nano Au particles at high temperatures. They revealed that the formed nano Au-ZrOx interface was the active site of CO2 electrochemical reduction.
"This electrochemical activation method can be used to develop other catalysts with nano metal-oxide interfaces," said Prof. ZHU.
This work was supported by the Strategic Priority Research Program of CAS, Liaoning Revitalization Talents Program, Youth Innovation Promotion Association of CAS, and Natural Science Foundation of China. (Text by ZHANG Lixiao)