Chemical reactions that occur on the surface of solid catalytic materials obey the Sabatier principle, that is, when the catalyst is too reactive, the total reaction rate is limited by elementary steps, for instance of desorption, diffusion, and recombination, eventually resulting in low activity.
While if the reactivity of a catalyst is too weak, the reactants are difficult to adsorb and dissociated, which will also result in relatively low reaction rate.
Therefore, a moderate reactivity is optimal to achieve the best reaction rate.
Recently, a group led by Prof. XIAO Jianping Xiao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. HOU Yang from Zhejiang University and Prof. WU Gang from the State University of New York at Buffalo studied the mechanism of electrocatalytic reduction of carbon dioxide to formic acid.
The result was recently published in Advanced Materials on Nov. 30.
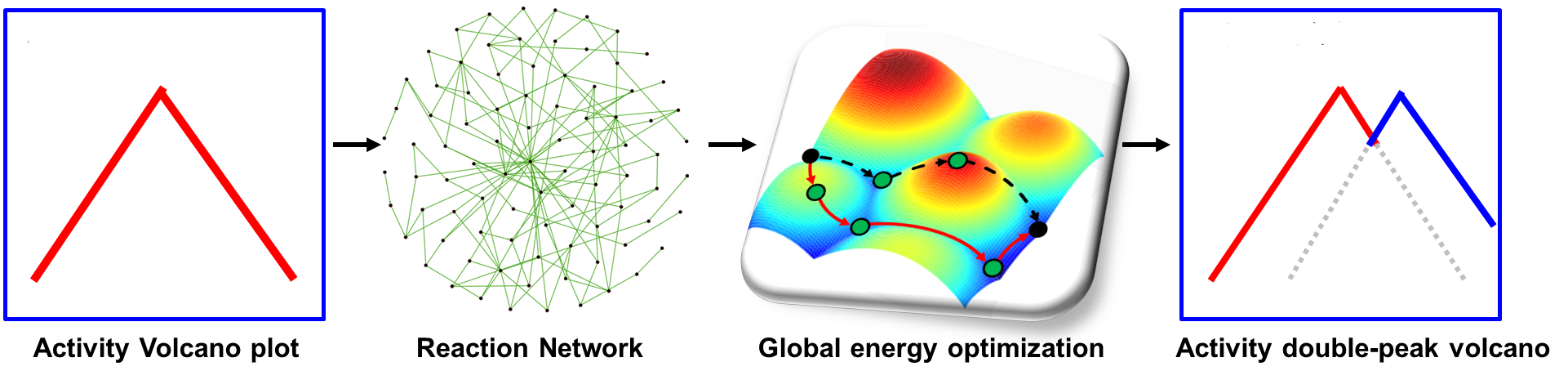
The map of activity rate → reaction network → global energy optimization → double-peak activity (Image by DONG Xue)
The scientists proved that the process of formic acid production in electrocatalytic reduction of carbon dioxide proceed via the formate (HCOO*) pathway, where carbon dioxide could be first protonated to formate (HCOO*), then the resulting formate was protonated to produce formic acid (HCOOH).
Previously, it was generally believed that CO was the only product via the path of carboxyl (COOH*). However, in this study, the scientists demonstrated that formic acid could also be produced through the carboxyl (COOH*) pathway on many metal surfaces at low potential by the theoretical results. And it explained the exceptional experimental results observed over Pd according to the analysis of the reaction network and the energy global optimization aspect.
"We also revealed in general that a reaction with very complex reaction networks, a traditional volcano curve with a single optimum should not be the case. Instead, an activity volcano map with multiple optimums should be general features," said Prof XIAO.
This work was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences and the Liaoning Revitalization Talents Program. (Text by DONG Xue)