Research News
-
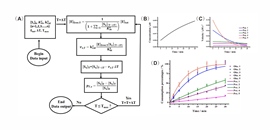 12 12, 2016Researchers in DICP Made New Progress in Study of Enzymatic Kinetics in Complex System
12 12, 2016Researchers in DICP Made New Progress in Study of Enzymatic Kinetics in Complex System
Numerous substrates could be identified when mass spectrometry-based proteomics is used to monitor the enzymatic reactions occurred in complex system with the presence of thousands of substrates. However, prioritizing new substrates is usually not achieved because the lacking of validated kinetic theory for such a complex system.
The research team headed by Prof. ZOU Hanfa and YE Mingliang from Dalian Institute of Chemical Physics (DICP) developed and validated kinetics models to facilitate understanding of enzyme kinetics in complex system on the basis of previous work. Especially, the ratio of substrate depletion was found to be independent of other coexisted substrates when the total substrate concentration is relative low. Based on this, the specificity constants, the enzymological constants reflecting the relative rates of an enzyme acting on different substrates, of numerous protease substrates presented in the complex system were accurately determined.
Enzyme specificity is fundamental in physiology, as metabolism would be impossible if enzymes could not distinguish between strictly similar substrates, and all reactions proceeded unchecked toward thermodynamic equilibrium. A significant contribution of this study is that it provides a theory to determine the enzyme specificity in high throughput. This work is published as an article in Molecular & Cellular Proteomics (doi:10.1074/mcp.M116.062869).(Text and Imaged by DENG Zhenzhen)
-
 12 12, 2016The Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou UniversityThe Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou University
12 12, 2016The Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou UniversityThe Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou University
Under the coordination of the Prof. YANG Shengli (Academician) and Prof. XU Guowang, on December 8th, 2016, 11 experts of Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences in the translational medical field visited the First Affiliated Hospital of Zhengzhou University (FAHZU) for the annual-conference of the cooperative projects. More than 50 clinical physicians from FAHZU including the President KAN Quancheng, the secretary ZHANG Shuijun, and the vice Presidents LIU Zhangsuo and WEN Jianguo et al. participated in the conference.
President KAN warmly greeted the guests and looked forward to the success of bilateral cooperation. Under the chair of Prof. ZHANG Yi and Prof. XU Guowang, the coordinators and scientists of 13 cooperation projects reported the progress of the related research work. After Prof. XU briefly introduced four platforms including proteomics, metabolomics, microfluidic microarrays and translational medicine in DICP, the clinical team of FAHZU introduced their new research subjects and clinical requirements, and two sides of scientists discussed the future possible cooperation fields. Finally, Prof. YANG Shengli summarized the conference, he encouraged the scientists of DICP to select research subjects from clinic practice and strengthen the integration of multiple omics platforms to meet the clinical needs and contribute to the health improvement.
This conference was greatly successful and got a very warm exchange atmosphere. All participants showed a strong enthusiasm to discuss the interested translational medicine research subjects. It can be expected that the cooperation between DICP and FAHZU will have a bright future. (Text and Imaged by YU Hao)
-
 12 12, 2016The Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou UniversityThe Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou University
12 12, 2016The Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou UniversityThe Translational Medicine Research Team of DICP Visited the First Affiliated Hospital of Zhengzhou University
Under the coordination of the Prof. YANG Shengli (Academician) and Prof. XU Guowang, on December 8th, 2016, 11 experts of Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences in the translational medical field visited the First Affiliated Hospital of Zhengzhou University (FAHZU) for the annual-conference of the cooperative projects. More than 50 clinical physicians from FAHZU including the President KAN Quancheng, the secretary ZHANG Shuijun, and the vice Presidents LIU Zhangsuo and WEN Jianguo et al. participated in the conference.
President KAN warmly greeted the guests and looked forward to the success of bilateral cooperation. Under the chair of Prof. ZHANG Yi and Prof. XU Guowang, the coordinators and scientists of 13 cooperation projects reported the progress of the related research work. After Prof. XU briefly introduced four platforms including proteomics, metabolomics, microfluidic microarrays and translational medicine in DICP, the clinical team of FAHZU introduced their new research subjects and clinical requirements, and two sides of scientists discussed the future possible cooperation fields. Finally, Prof. YANG Shengli summarized the conference, he encouraged the scientists of DICP to select research subjects from clinic practice and strengthen the integration of multiple omics platforms to meet the clinical needs and contribute to the health improvement.
This conference was greatly successful and got a very warm exchange atmosphere. All participants showed a strong enthusiasm to discuss the interested translational medicine research subjects. It can be expected that the cooperation between DICP and FAHZU will have a bright future. (Text and Imaged by YU Hao)
-
 12 08, 2016DICP Researchers Develop a Highly Specific Ratiometric Two-photon Fluorescent Probe to Detect Dipeptidyl Peptidase IV in Plasma and Living SystemsThe Pharmaceutical Resource Discovery Group led by Prof. GE Guangbo and Prof. YANG Ling in DICP developed a highly specific ratiometric two-photon fluorescent probe GP-BAN. It was successfully used for detection of dipeptidyl peptidase IV in plasma and two-photon imaging of endogenous DPP-IV in living cells and tissues.
12 08, 2016DICP Researchers Develop a Highly Specific Ratiometric Two-photon Fluorescent Probe to Detect Dipeptidyl Peptidase IV in Plasma and Living SystemsThe Pharmaceutical Resource Discovery Group led by Prof. GE Guangbo and Prof. YANG Ling in DICP developed a highly specific ratiometric two-photon fluorescent probe GP-BAN. It was successfully used for detection of dipeptidyl peptidase IV in plasma and two-photon imaging of endogenous DPP-IV in living cells and tissues.
Dipeptidyl peptidase IV (DPP-IV, CD26) is a multifunctional serine protease enzyme with key roles in the control of endocrine and immune function, cell metabolism, growth and adhesion. It is a famous therapeutic target for the treatment of type-2 diabetes. DPP-IV also exhibits co-stimulatory role in T-cell activation, adhesion to extracellular matrix proteins, and serves as the functional receptor for Middle East respiratory syndrome coronavirus infection.
DPP-IV now has becoming the center of attention as a novel molecular marker or a potential therapeutic target for cancer. Furthermore, increasing evidence has demonstrated that the abnormal presence or altered level of DPP-IV in plasma can serve as a potential biomarker for early diagnosis and prognosis evaluation of many diseases. Thus, the accurate measurement of DPP-IV in complex biological samples, especially in plasma, is of great importance for disease diagnosis, drug discovery and clinical practice.
The Pharmaceutical Resource Discovery Group led by Prof. GE Guangbo and Prof. YANG Ling in DICP developed a highly specific ratiometric two-photon fluorescent probe GP-BAN. It was successfully used for detection of dipeptidyl peptidase IV in plasma and two-photon imaging of endogenous DPP-IV in living cells and tissues.
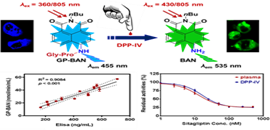
Figure. Proposed response mechanism of the GP-BAN/BAN system for DPP-IV detection (Imaged by ZOU Liwei)
In this study, Gly-Pro-BAN (GP-BAN) was designed on the basis of the catalytic properties and substrate preference of DPP-IV. It could be readily hydrolyzed upon addition of DPP-IV under physiological conditions. GP-BAN displayed good reactivity and high selectivity towards DPP-IV over other human serine hydrolases including FAP, DPP-VIII, and DPP-IX. It was evidenced by both reaction phenotyping and inhibition assays. Furthermore, GP-BAN was successfully used to monitor the real activities of DPP-IV in complex biological systems including tissue preparations, plasma and living cells.
Meanwhile, GP-BAN could serve as a promising tool for high-throughput screening of DPP-IV inhibitors by using human plasma and tissue preparations as enzyme source. This is the first report to screen DPP-IV inhibitors by using readily available human plasma as enzyme source. GP-BAN was also successfully used for two-photon imaging of endogenous DPP-IV in living cells and tissues, and showed low cytotoxicity, high ratiometric imaging resolution and deep-tissue penetration ability. Thus, the fluorescent probe GP-BAN could serve as a promising imaging tool to explore the biological functions and physiological roles of this key enzyme in living systems.
The related results were published in Biosensors and Bioelectronics. (DOI: 10.1016/j.bios.2016.11.068).This work was supported by Natural Science Foundation of China and National Basic Research Program of China. (Text and Imaged by ZOU Liwei)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201,
E-mail: luxinyi@dicp.ac.cn
-
 12 08, 2016DICP Researchers Develop a Highly Specific Ratiometric Two-photon Fluorescent Probe to Detect Dipeptidyl Peptidase IV in Plasma and Living SystemsThe Pharmaceutical Resource Discovery Group led by Prof. GE Guangbo and Prof. YANG Ling in DICP developed a highly specific ratiometric two-photon fluorescent probe GP-BAN. It was successfully used for detection of dipeptidyl peptidase IV in plasma and two-photon imaging of endogenous DPP-IV in living cells and tissues.
12 08, 2016DICP Researchers Develop a Highly Specific Ratiometric Two-photon Fluorescent Probe to Detect Dipeptidyl Peptidase IV in Plasma and Living SystemsThe Pharmaceutical Resource Discovery Group led by Prof. GE Guangbo and Prof. YANG Ling in DICP developed a highly specific ratiometric two-photon fluorescent probe GP-BAN. It was successfully used for detection of dipeptidyl peptidase IV in plasma and two-photon imaging of endogenous DPP-IV in living cells and tissues.
Dipeptidyl peptidase IV (DPP-IV, CD26) is a multifunctional serine protease enzyme with key roles in the control of endocrine and immune function, cell metabolism, growth and adhesion. It is a famous therapeutic target for the treatment of type-2 diabetes. DPP-IV also exhibits co-stimulatory role in T-cell activation, adhesion to extracellular matrix proteins, and serves as the functional receptor for Middle East respiratory syndrome coronavirus infection.
DPP-IV now has becoming the center of attention as a novel molecular marker or a potential therapeutic target for cancer. Furthermore, increasing evidence has demonstrated that the abnormal presence or altered level of DPP-IV in plasma can serve as a potential biomarker for early diagnosis and prognosis evaluation of many diseases. Thus, the accurate measurement of DPP-IV in complex biological samples, especially in plasma, is of great importance for disease diagnosis, drug discovery and clinical practice.
The Pharmaceutical Resource Discovery Group led by Prof. GE Guangbo and Prof. YANG Ling in DICP developed a highly specific ratiometric two-photon fluorescent probe GP-BAN. It was successfully used for detection of dipeptidyl peptidase IV in plasma and two-photon imaging of endogenous DPP-IV in living cells and tissues.
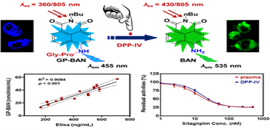
Figure. Proposed response mechanism of the GP-BAN/BAN system for DPP-IV detection (Imaged by ZOU Liwei)
In this study, Gly-Pro-BAN (GP-BAN) was designed on the basis of the catalytic properties and substrate preference of DPP-IV. It could be readily hydrolyzed upon addition of DPP-IV under physiological conditions. GP-BAN displayed good reactivity and high selectivity towards DPP-IV over other human serine hydrolases including FAP, DPP-VIII, and DPP-IX. It was evidenced by both reaction phenotyping and inhibition assays. Furthermore, GP-BAN was successfully used to monitor the real activities of DPP-IV in complex biological systems including tissue preparations, plasma and living cells.
Meanwhile, GP-BAN could serve as a promising tool for high-throughput screening of DPP-IV inhibitors by using human plasma and tissue preparations as enzyme source. This is the first report to screen DPP-IV inhibitors by using readily available human plasma as enzyme source. GP-BAN was also successfully used for two-photon imaging of endogenous DPP-IV in living cells and tissues, and showed low cytotoxicity, high ratiometric imaging resolution and deep-tissue penetration ability. Thus, the fluorescent probe GP-BAN could serve as a promising imaging tool to explore the biological functions and physiological roles of this key enzyme in living systems.
The related results were published in Biosensors and Bioelectronics. (DOI: 10.1016/j.bios.2016.11.068).This work was supported by Natural Science Foundation of China and National Basic Research Program of China. (Text and Imaged by ZOU Liwei)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201,
E-mail: luxinyi@dicp.ac.cn
-
 12 02, 2016DICP Researchers Prepare a New Oxygen-permeable Ceramic Membrane for Hydrogen SeparationProf. YANG Weishen and Prof. ZHU Xuefeng from State Key Laboratory of Catalysis in DICP developed a new method for hydrogen separation by using an oxygen-permeable ceramic membrane. It' s successfully demonstrated by experiments with excellent performance. The hydrogen separation rate is high up to 16.3 mL cm-2 min-1 with separation factor up to >10,000. This separation rate is 2-3 magnitudes higher than the proton conducting membranes, and comparable to Pd-based metallic membranes. Besides, no performance degradation was observed in a long-term operation with the feeding 200 ppm H2S containing gas. The experiment results indicate that the membranes exhibit high selectivity, high hydrogen separation rate and stability under H2S-containing atmosphere. It could be used to produce high-purity or ultra-high-purity hydrogen for fuel cells, semiconductor manufacturing, photovoltaic cells production, etc.
12 02, 2016DICP Researchers Prepare a New Oxygen-permeable Ceramic Membrane for Hydrogen SeparationProf. YANG Weishen and Prof. ZHU Xuefeng from State Key Laboratory of Catalysis in DICP developed a new method for hydrogen separation by using an oxygen-permeable ceramic membrane. It' s successfully demonstrated by experiments with excellent performance. The hydrogen separation rate is high up to 16.3 mL cm-2 min-1 with separation factor up to >10,000. This separation rate is 2-3 magnitudes higher than the proton conducting membranes, and comparable to Pd-based metallic membranes. Besides, no performance degradation was observed in a long-term operation with the feeding 200 ppm H2S containing gas. The experiment results indicate that the membranes exhibit high selectivity, high hydrogen separation rate and stability under H2S-containing atmosphere. It could be used to produce high-purity or ultra-high-purity hydrogen for fuel cells, semiconductor manufacturing, photovoltaic cells production, etc.
The hydrogen separation and purification technology is significantly important for the applications of hydrogen in various fields. Compared to other methods for hydrogen separation, inorganic dense hydrogen-permeable membranes exhibit 100% permeation selectivity for hydrogen. So they are regarded as one of the most promising methods for high-purity hydrogen production. However, the state-of-art Pd-based metallic membranes are limited by the scarce resource of Pd and low stability under H2S-containing atmosphere. In addition, another type of inorganic dense hydrogen-permeable membranes, i.e. proton conducting membranes, exhibit low hydrogen permeability and are also easily poisoned by H2S.
To solve above problems, Prof. YANG Weishen and Prof. ZHU Xuefeng from State Key Laboratory of Catalysis in Dalian Institute of Chemical Physics developed a new method for hydrogen separation by using an oxygen-permeable ceramic membrane. It's successfully demonstrated by experiments with excellent performance. The hydrogen separation rate is high up to 16.3 mL·cm-2·min-1 with separation factor up to >10,000. This separation rate is 2-3 magnitudes higher than the proton conducting membranes, and comparable to Pd-based metallic membranes. Besides, no performance degradation was observed in a long-term operation with the feeding 200 ppm H2S containing gas. The experiment results indicate that the membranes exhibit high selectivity, high hydrogen separation rate and stability under H2S-containing atmosphere. It could be used to produce high-purity or ultra-high-purity hydrogen for fuel cells, semiconductor manufacturing, photovoltaic cells production, etc.
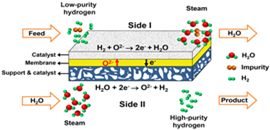
The concept of hydrogen separation by using the oxygen-permeable ceramic membrane (Imaged by LI Wenping)
The figure shows the concept of hydrogen separation by using the oxygen-permeable ceramic membrane. The low-purity hydrogen is fed to one side of the membrane (Side I) and the steam is fed to the other side of the membrane (Side II). On the Side II, water is spited into hydrogen and oxygen ions at elevated temperature. And then oxygen ions diffuse to the Side I and react with hydrogen to produce water. Therefore, the high-purity hydrogen is acquired after condensation and drying of the outlet gas from Side II. The amount of hydrogen produced from Side II is equivalent to that consumed on Side I. As a whole, there is no net chemical reaction occurring, but a mass exchange is achieved between the two sides. Thus, it is reasonable to regard this process as a hydrogen separation process with the help of steam.
The related results were published in Energy Environ. Sci. (DOI: 10.1039/C6EE02967A). This work was supported by Natural Science Foundation of China,Chinese Academy of Sciences and DICP DMTO project. (Text and Imaged by LI Wenping)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201,
E-mail: luxinyi@dicp.ac.cn