In a study published in Angewandte Chemie International Edition, a research team led by Profs. BAO Xinhe, GAO Dunfeng, and ZHANG Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), along with Prof. WANG Guoxiong from Fudan University, achieved efficient bicarbonate-mediated integrated carbon dioxide (CO2) capture and electrolysis to CO through an ionomer-driven reaction microenvironment control strategy.
Traditional CO2 capture and conversion routes from industrial flue gas typically follow a "capture-release-compression-electrolysis" tandem pathway. The bicarbonate-mediated integrated CO2 capture-electrolysis route, as an emerging reactive carbon capture technology, couples upstream CO2 capture with subsequent electrocatalytic conversion, reducing the energy consumption associated with obtaining high-purity CO2 feedstock.
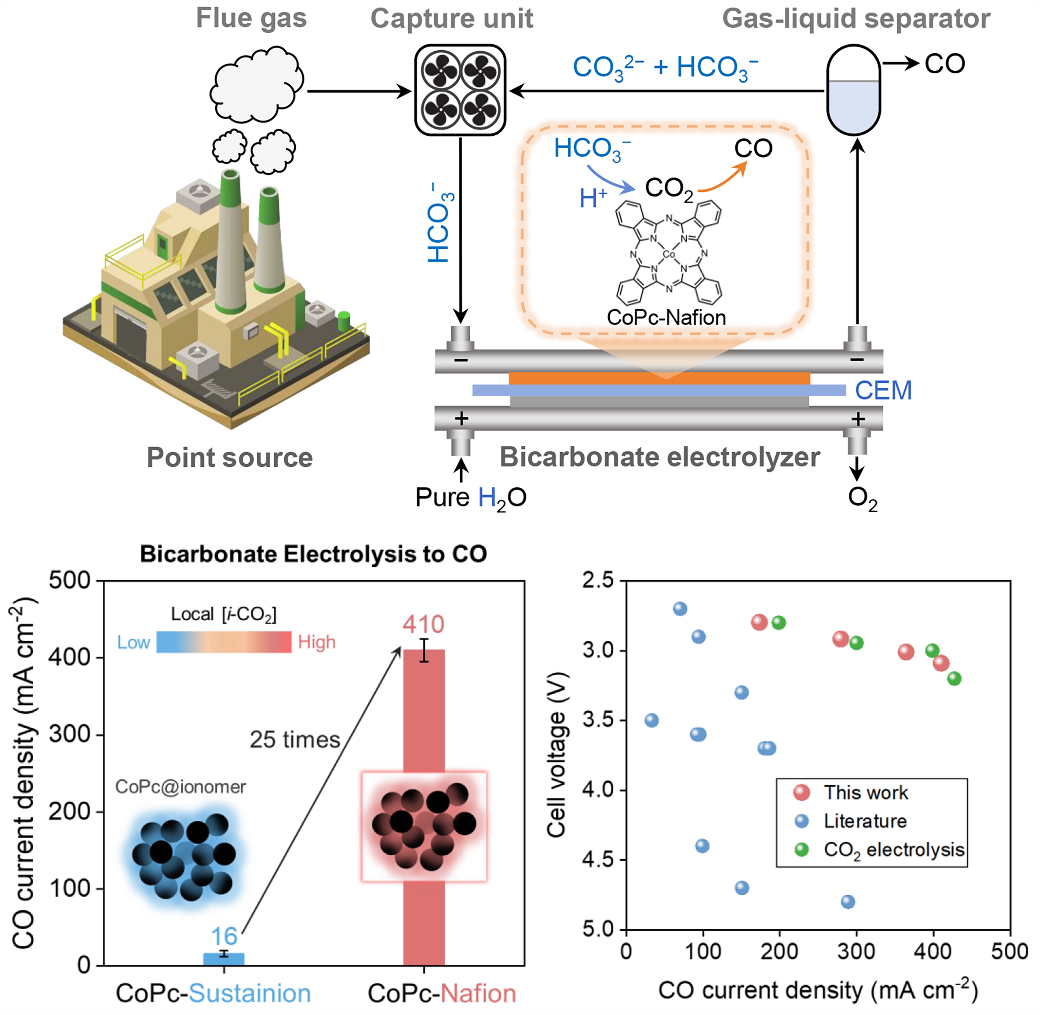
The electrolysis of bicarbonate capture liquids is a crucial step in the bicarbonate-mediated integrated CO2 capture-electrolysis route. However, this step suffers from insufficient current density (low reaction rate) and high cell voltage (low energy efficiency) .
Ionomer-driven reaction microenvironment control in bicarbonate-mediated integrated CO2 capture and electrolysis (Image by RONG Youwen)
In this study, the researchers manipulated the reaction microenvironments by introducing ionomers into cobalt phthalocyanine (CoPc) electrodes, improving the performance of bicarbonate electrolysis. In a cation exchange membrane-based zero-gap electrolyzer, the CoPc electrode modified with a Nafion ionomer exhibited a high CO Faradaic efficiency of 93% at an applied current density of 300 mA cm−2 and a CO partial current density of 410 mA cm−2 at a low cell voltage of 3.09 V.
Electrode structure characterization and finite element simulation results demostrated that the proton conductivity of the Nafion ionomer increased the local concentration of in situ generated CO2 (i-CO2) in the proximity of the CoPc catalyst, resulting in improved CO formation.
Furthermore, the researchers demonstrated a closed-loop CO2 capture and electrolysis cycle at the device level using the Nafion-incorporated CoPc electrode and a simulated flue gas.
"Our study shows the potential of the reaction microenvironment control strategy for improving bicarbonate electrolysis performance and advancing reactive carbon capture technology," said Prof. GAO.