The electrocatalytic reduction of carbon dioxide (CO2) into valuable chemicals and fuels provides a sustainable route to address global climate and energy challenges. However, CO2 electroreduction typically operates under alkaline or neutral conditions, where the carbonation side reaction causes carbon loss. Moreover, the main product— formate—requires additional post-treatment steps, such as acidification, to obtain formic acid.
Acidic CO2 electroreduction can effectively mitigate carbonation issues and directly produce formic acid, but harsh acidic environments often lead to metal leaching and rapid catalyst degradation, limiting both activity and long-term durability.
In a study published in Angewandte Chemie International Edition, a research team led by Prof. GAO Dunfeng, in collaboration with Prof. ZHOU Xukai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved durable acidic CO2 electroreduction to formic acid through a metal-phase protection strategy.
Metal-phase protection suppresses Bi leaching for durable acidic CO2 electroreduction to formic acid (Image by TAN Zijian)
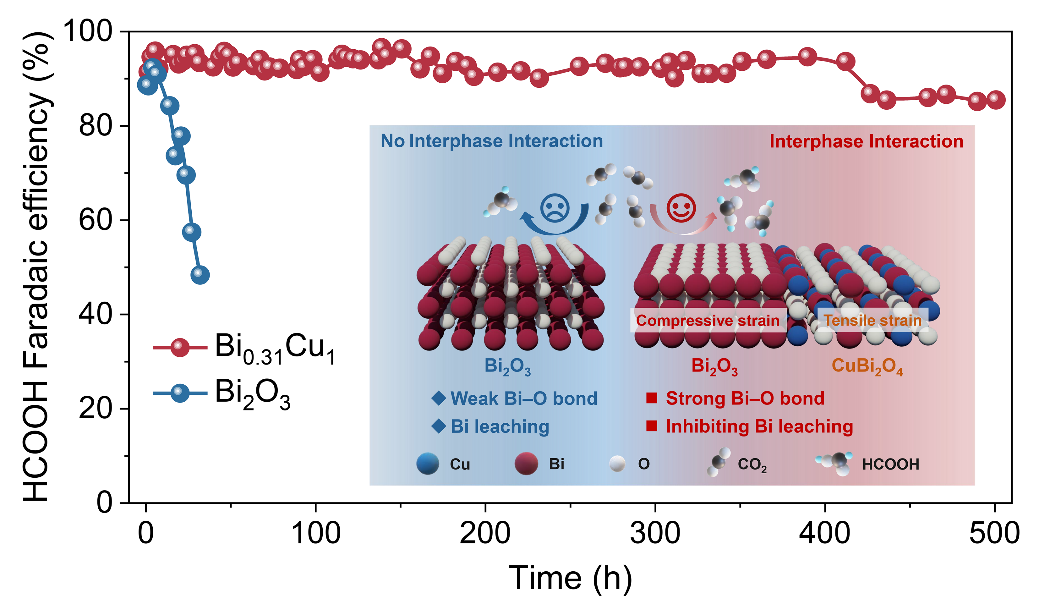
Researchers proposed a metal-phase protection strategy that enabled the in situ formation of a Bi-Cu bimetallic oxide catalyst (Bi0.31Cu1). This catalyst exhibits high activity and durability for acidic CO2 electroreduction to formic acid (HCOOH). The Bi0.31Cu1 catalyst delivers a Faradaic efficiency for HCOOH above 90% in a wide current density range from 200 to 650 mA cm−2. In a 0.5 M KCl electrolyte at pH 2, the Bi0.31Cu1 catalyst can continuously produce HCOOH with approximately 90% Faradaic efficiency at 200 mA cm−2 for 500 hours.
Furthermore, researchers revealed that the interphase interaction between the Bi2O3 phase and the CuBi2O4 phase within the Bi0.31Cu1 catalyst induces compressive strain and lattice contraction in the Bi2O3 phase. This strengthens the Bi−O bond, effectively suppressing Bi leaching during catalyst reconstruction and thus delivering both high activity and long-term durability. The general applicability of this metal-phase protection strategy was further demonstrated using a Bi-Mg bimetallic catalyst.
"Our study showcases the promise of the metal-phase protection strategy for developing highly efficient catalysts with long-term durability for acidic CO2 electrolysis," said Prof. GAO.