Efficient and controllable hydrogen activation through heterogeneous catalysis is crucial for producing clean energy and chemicals. However, the fragile nature of hydrogen species and the complexity of material surfaces make it challenging to precisely identify critical intermediate species, as well as their atom-scale conformations and locations. This necessitates the development of high-resolution in situ characterization techniques for accurate hydrogen detection.
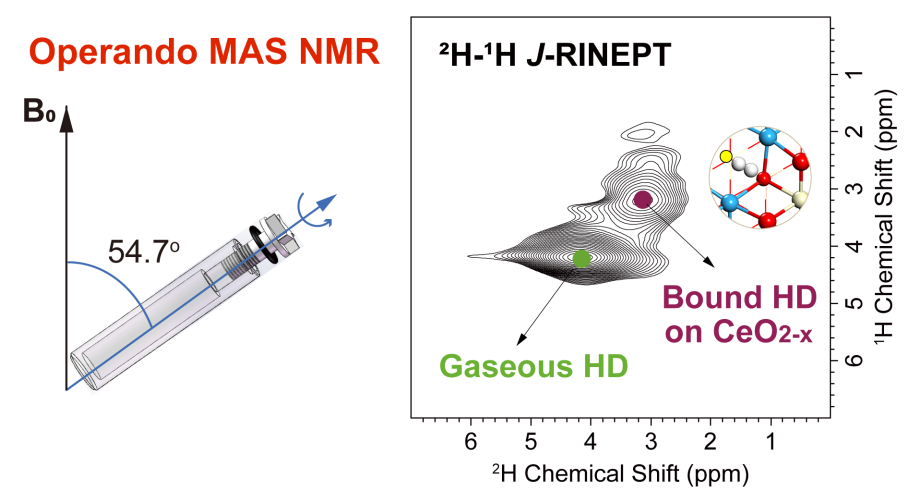
Solid-state magic-angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for analyzing surface species on heterogeneous catalysts, providing atomic-level structural information. However, conventional MAS NMR, which detects species remaining on solid catalysts after they have been separated from their reaction environment, is incapable of capturing volatile intermediates like activated hydrogen species.
Recently, a research team led by Prof. HOU Guangjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed an environment-sensitive nondissociative activated dihydrogen binding on the reduced ceria surface, closely resembling dihydrogen complexes in molecular science, using state-of-the-art high-pressure operando solid-state NMR. The study was published in Journal of the American Chemical Society.
Revealing the nondissociative activated dihydrogen binding on reduced ceria surface by high-pressure operando solid-state NMR spectroscopy (Image by YAO Xinlong and JI Yi)
The researchers fully utilized the high-pressure operando solid-state NMR technique, alongside a comprehensive set of H2/D2/HD adsorption experiments and various NMR spectroscopic methods, to investigate the states of activated hydrogen species on ceria surfaces.
The researchers found that the hydrogen species bound to the reduced ceria surfaces were primarily nondissociated dihydrogen species, resembling the dihydrogen complexes in homogeneous and enzymatic catalysis. They identified these nondissociated dihydrogen species as retaining their hydrogen-hydrogen bonds on the ceria surface. Additionally, they demonstrated that these species exhibited slightly reduced J-coupling and longer T1 relaxation times compared to gaseous molecules, confirming bond length stretching, reduced mobility, and their bound and activated nature.
Furthermore, the researchers and their collaborators showed that these bound dihydrogen species were confined by surface oxygen vacancies. Importantly, the proton isotropic chemical shifts were found to correlate with the degree of surface reduction, providing a high-resolution, nondestructive method for quantitatively analyzing the structure and catalytic functions of different catalyst surfaces.
"On one hand, our study bridges the gap between homogeneous organometallic catalysis and heterogeneous catalysis, offering valuable insights into the hydrogenation mechanism and potentially enhancing hydrogen utilization," said Prof. HOU."On the other hand, the analytic approach presented here can be applied to analyze the adsorption and activation processes of many other volatile molecules, such as CH4, CO, and CO2, benefiting the rational design of more efficient catalysts."
The above work was financially supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the DICP Innovation Foundation.