Metal hydrides (M-H), critical but ubiquitous intermediates in a broad variety of catalytic reactions, are important in the field of heterogeneous catalysis. However, the comprehensive characterization and understanding of M-H species are still challenging.
Recently, a research team led by Prof. HOU Guangjin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the reactive gallium-hydrogen (Ga-H) species on the surface of gallium oxide (Ga2O3) by using solid-state nuclear magnetic resonance (ssNMR).
This study was published in the Journal of the American Chemical Society on Sep. 14.
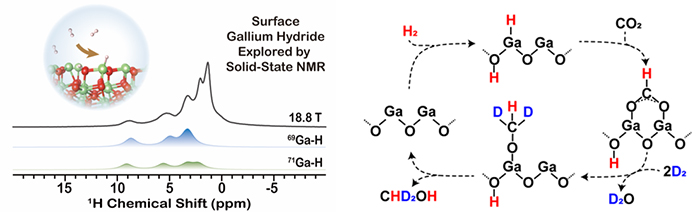
The ssNMR spectroscopic evidence of surface gallium hydride on Ga2O3, and the nature of reactivity of this unique species in CO2 hydrogenation (Image by CHEN Hongyu and Gao Pan)
The researchers provided ssNMR evidence of surface Ga-H species generated on a practical nano Ga2O3 oxide catalyst during direct H2 activation and propane dehydrogenation reactions.
They found that the complex 1H NMR signature of Ga-H species came from strong J and dipolar/quadrupolar couplings using NMR techniques and numerical simulations. And they revealed comprehensive information on the structural configuration and formation mechanism of this special M-H species with complementary NMR and DFT analysis.
Furthermore, they used 13CO2 adsorption experiment to prove that Ga-H species were the key intermediates in the hydrogenation process of CO2.
"The analytic approach presented in this study can be extended to other M-H analysis, and it may benefit the design of more efficient Ga-based catalysts," said Prof. HOU.
The above work was supported by the National Key R&D Programme of China, the National Natural Science Foundation of China, the Liaoning Revitalization Talents Programme, the China National Postdoctoral Programme for Innovative Talents, and the China Postdoctoral Science Foundation. (Text by CHEN Hongyu and GAO Pan)